Background and overview[1]
α-Methylbenzonitrile, also called 2-phenylpropionitrile, can be used to synthesize 2-phenylpropionic acid, which is widely used in the synthesis of medicines, spices and pesticides.
Preparation[1]
Currently, the industrial production of α-methylbenzonitrile is mainly produced through the methylation reaction of phenylacetonitrile.
The literature (Synthesis, 2018, 50(15): 2878-2886.) used phenylacetonitrile as raw material, methyl iodide as methylating reagent, and synthesized α-methylbenzene under the catalysis of lithium diisopropylamide. Nitrile.
The literature (Chinese Journal of Pharmaceutical Industry, 1991 (1): 2-4.) uses dimethyl sulfate as the methylation reagent, and reacts at room temperature under the catalysis of sodium hydroxide and tetrabutylammonium bromide. In 4h, α-methylbenzonitrile was generated with a yield of 72%.
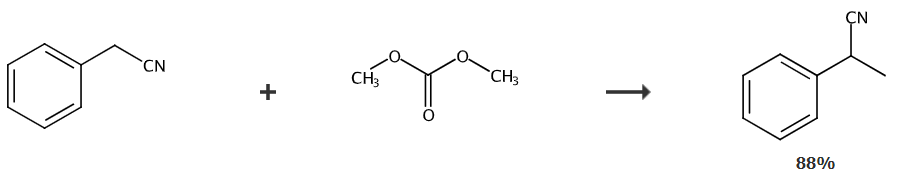
The literature (Organic Syntheses. John Wiley & Sons, Inc. 2003: 169-175.) uses dimethyl carbonate as the methylating reagent, and combines phenylacetonitrile, dimethyl carbonate, and potassium carbonate in a molar ratio of 1:16.4:2.1 After mixing, the mixture was heated to 180°C in an autoclave and reacted for 18 hours to obtain α-methylbenzonitrile with a yield of 93%.
CN201811310926.5 provides a phenylacetonitrile methylation method based on pressurized distillation technology, including the following steps:
1). Mix phenylacetonitrile, sodium alkoxide and dimethyl carbonate (dimethyl carbonate also serves as methylation reagent and solvent) according to the molar ratio of 1:0.1~0.4:4~10, and mix at 2± 0.2MPa pressure, 160~200℃ reaction temperature, 4~8h reaction;
2) After the reaction is completed, the temperature is lowered (100±5°C) to normal pressure distillation, then vacuum distillation (20mmHg), and the fractions between 108 and 113°C are collected to obtain α-methylbenzonitrile as the product .
Apply[2]
α-Methylbenzonitrile can be used to prepare 2-phenylpropionic acid. CN201410527921.3 reports a synthesis method of 2-phenylpropionic acid, which belongs to the field of medicine. α-Methyl benzonitrile undergoes alkaline hydrolysis and acidification to obtain 2-phenylpropionic acid. The crude product is distilled under reduced pressure of 3 mmHg to obtain high-purity 2-phenylpropionic acid with an HPLC purity greater than 99%. The use of precious metal complexes is avoided during the entire reaction process, and the process is simple and easy to operate.
Main reference materials
[1] [Chinese invention] CN201811310926.5 Methylation method of phenylacetonitrile based on pressure distillation technology
[2]CN201410527921.3 A kind of preparation method of 2-phenylpropionic acid

 微信扫一扫打赏
微信扫一扫打赏

