Background and overview[1]
1,3-Diphenylurea is a urea compound. Urea refers to a compound containing R1R2N-CO-NR1R2 functional group. It is an artificial The first type of organic compound synthesized. Due to its very good reactivity, urea is an important synthon in synthetic chemistry. Urea has excellent biological activity and is widely found in natural products, drugs and pesticide molecules. In addition, urea is also used as a protein inhibitor in protein chemistry.
Preparation[1-2]
Report 1,
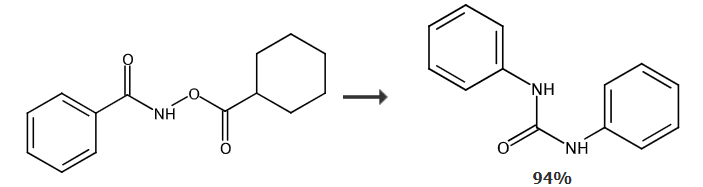
Synthesis of 1,3-diphenyl urea from N-cyclohexyloxybenzamide: At room temperature and in an air atmosphere, add [RuCl2 in sequence in a dry reaction tube (p-cymene))]2 (0.005), N-cyclohexanoyloxybenzamide (0.2mol), silver acetate (AgOAc) (0.4mol) and dioxane solvent (dioxane) (1mL), then the temperature was raised to 80°C and reacted for 12 hours. After the reaction was completed, the product was separated by column chromatography with a yield of 94%.
Report 2,

Aniline (37.6 mg, 0.4 mmol) was dissolved in acetonitrile (1.5 mL) and placed in a 25 ml reaction tube. Use a rubber stopper to seal the reaction tube and add CF3SO3CF3 (43.6 mg, 0.2 mmol) was dissolved in 0.5 mL acetonitrile and injected into the reaction tube with a syringe. The mixed liquid was stirred at room temperature for 48 hours. After the reaction was completed, the reaction was quenched with 2 drops of water and the pressure was reduced. The solvent was removed, petroleum ether: ethyl acetate = 5:1 (v/v) was used as the eluent, and the product was obtained by column chromatography: 1,3-diphenylurea (white solid, 38.3 mg), yield: 90% . 1H NMR (500MHz, DMSO-d6): δ8.65 (s, 2H), 7.45 (d, J=8.0Hz, 4H), 7.28 (t, J=7.8Hz, 4H) , 6.96 (t, J=7.3Hz, 2H). 13C NMR (126MHz, DMSO-d6): δ153.0, 140.2, 129.3, 122.3, 118.7.
Report 3,
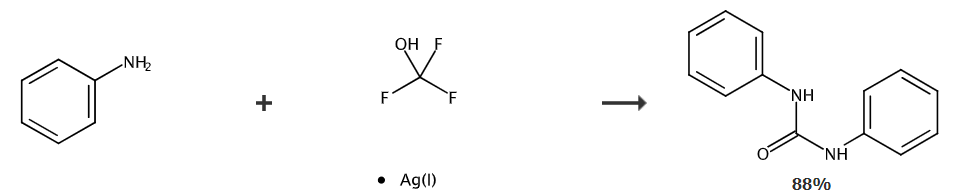
Under nitrogen protection, aniline (37.6 mg, 0.4 mmol) was dissolved in dichloromethane (1.5 mL) and placed in a 25 ml reaction tube. Use a rubber stopper to seal the reaction tube. The reaction system was cooled to -80°C and 0.5 mL AgOCF was added. 3 (0.2mmol) acetonitrile solution was injected into the reaction tube with a syringe. The mixed liquid naturally returned to room temperature while stirring for 12 hours. After the reaction was completed, the reaction was quenched with 2 drops of water. The solvent was removed under reduced pressure and used Petroleum ether: ethyl acetate = 5:1 (v/v) was used as the eluent. The product was obtained by column chromatography: 1,3-diphenyl urea (white solid, 37.4 mg), yield: 88%.
References
[1] [Chinese invention] CN201811512934.8 An N,N’-disubstituted urea compound and its synthesis method
[2] [Chinese invention] CN201910248348.5 A simple preparation method of N-acyl compounds

 微信扫一扫打赏
微信扫一扫打赏

