Background and overview[1]
2-Hydroxyterephthalic acid (HTA), as a BB-type polymer monomer, can be used in the modification of high-performance fiber materials – poly-p-phenylene benzoxadiazole (PBX), using The hydrogen bonds and surface polarity generated by its hydroxyl groups improve the material’s axial compression resistance and composite bonding properties. Based on the research on AB-type polymerized monomers by the Zhejiang University of Technology research team, it was found that HTA has potential application prospects in the preparation of new AB-type monomers for ordered polymers, such as hydroxyl-modified poly-p-phenylene benzo Bioxazole (PBO) and poly(p-phenylene terephthalamide) (PPTA), etc. In addition, as an important organic intermediate, HTA also has certain application value in medicine and functional materials. Compared with the bulk product terephthalic acid (TPA), the preparation of HTA is mostly conducted on a laboratory scale at home and abroad, and there are still no industrial production and commercial reports. Therefore, there is a need for research on the synthesis of HTA and its valuable industrialization routes. Very important meaning. There are semi-synthetic methods and chemical synthesis methods for the preparation of HTA. The semi-synthetic method first uses terephthalic acid biological wild-type strains to prepare 1,2-dihydroxy-3,5-diene-1,4-cyclohexanedicarboxylic acid. , and then use chemical methods to remove the 1-hydroxyl group by heating in dilute sulfuric acid to generate HTA, which is a combination of biological and chemical synthesis. There are many foreign studies on the preparation of HTA by chemical synthesis, but there are few reports in China. The methods involved include: saponification and hydrolysis of dimethyl aminoterephthalate, diazo and then acidic hydrolysis; 2-hydroxy-p-xylene is protected by hydroxyl group first. Methyl oxidation method of post-oxidation and deprotection; cyanohydrolysis method of the ester group of dimethyl terephthalate and hydroxybenzonitrile; catalytic hydrolysis method of brominated terephthalic acid (BTA); (trifluoromethyl) High-pressure carboxylation method of CO2 of hydroxybenzoic acid; high-pressure carboxylation method of CO2 of m-hydroxybenzoic acid (HBA) in the presence of SiO2.
Preparation[1]
1) Catalytic hydrolysis of 2-bromoterephthalic acid
Into a 100mL four-necked flask, add 0.89g (3.6mmol) of homemade BTA with a purity of 95.6%, 0.87g NaOH (21.8mmol), 0.10g copper powder (1.6mmol) and 16.6mL water. Stir, heat and raise the temperature to 100°C for reflux reaction for 11.5 hours (TLC controls the reaction time). After the reaction solution is cooled, filter out the copper powder. Add 10% hydrochloric acid dropwise to the filtrate while stirring to precipitate, suction filtrate, wash with water, and dry to obtain 0.42g of hydrolyzed solution. The product, HPLC purity was 96.65%, and the yield was 63.27%. Melting point 314~322℃.
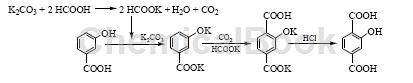
2) m-Hydroxybenzoic acid CO2 carboxylation method
Prepare carboxylation solvent HCOOK. Add 140.3g K2CO3 (1.015mol) and 70mLH2O into a 500mL four-necked flask and stir evenly. Add 110.0g HCOOH (85%, 2.03mol) dropwise. After adding, heat up to reflux and stir for 1 hour. , distill and dehydrate under reduced pressure until the kettle temperature is above 200°C. After cooling, it becomes potassium formate (melting point 167~170°C). Raise the temperature of the HCOOK prepared above to above 180°C. Under effective stirring, add 80.0g HBA (0.579 mol) at one time, pass CO2, and slowly add 96.0g K2CO3 (0.695 mol) in batches at 180~190°C (0.5~1.0h). mol), after adding, raise the temperature to 230°C, stir the reaction for 6 hours in the presence of CO2, wait until it is cooled to 60°C, slowly add it to 500mL of 0.1% NaHSO3 hot water solution, stir until completely dissolved, then add concentrated hydrochloric acid dropwise to adjust the pH value to neutral , add activated carbon for decolorization and filtration, adjust the filtrate to pH=1~2 with concentrated hydrochloric acid, filter, wash and dry to obtain 101.6g of crude product HTA, with a crude product yield of 96.3%. Add the obtained crude HTA to 7.5 times of water (mass ratio), neutralize it with concentrated ammonia water under stirring until it becomes neutral. After it is completely dissolved, add activated carbon for decolorization and adsorption filtration. The filtrate is adjusted to pH=1~2 with concentrated hydrochloric acid, cooled and filtered. The filter cake was then beaten and washed with 300mL of 10% hydrochloric acid, filtered and washed with water to obtain 70g of dried HTA primary refined product (HPLC purity 96.8%, K+376mg/kg), with a total yield of 66.4%; it was then refined twice in the same way, 110 After drying at ℃, 62.1g of HTA polymer grade monomer (HPLC purity 99.7%, metal ion K+21mg/kg, Na+8mg/kg) was obtained. The melting point was 314~317℃. The total yield based on HBA was 58.9%.
Main reference materials
[1] New technology for the synthesis of high-purity 2-hydroxyterephthalic acid

 微信扫一扫打赏
微信扫一扫打赏

