Background and overview[1]
2,6-Dimethylchloroacetanilide can be used as a pharmaceutical synthesis intermediate. If 2,6-dimethylchloroacetanilide is inhaled, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, wash the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
2,6-Dimethylchloroacetanilide is prepared as follows:
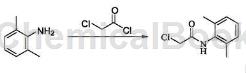
Dissolve 10g sodium carbonate (0.1mol) in water, add 200ml toluene after dissolution, add 10g 2,6-diethylaniline (0.068mol), add 7.7g chloroacetyl chloride (0.068mol) dropwise in an ice bath, Stir at room temperature for 2 hours. TLC monitors the reaction to be completed. The reaction mixture is filtered, washed with water and petroleum ether to obtain a pink solid (VII-1). Melting point 122-124℃.
Apply[2]
2,6-Dimethylchloroacetanilide can be used as an intermediate in pharmaceutical synthesis, such as the preparation of ranolazine intermediate. Ranolazine is a new anti-angina drug researched and developed by Syntex in the United States, 2006 It has been approved by the FDA and is now available in the United States for the treatment of chronic angina. It can provide myocardial protection at the cellular level by improving myocardial energy metabolism without affecting heart rate, blood pressure and hemodynamics, and has good application prospects. The specific reactions are as follows:
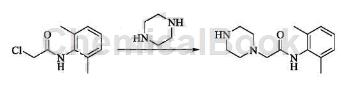
Into a four-necked flask equipped with mechanical stirring, add 500g of 1-formylpiperazine, 866g of 2-chloro-N-(2,6-dimethylphenyl)acetamide, 908g of potassium carbonate, and 2500mL of acetone. , stir for 8 hours at 25°C, filter, concentrate and dry the filtrate, add 1500ml of dichloromethane and 1000ml of deionized water, let stand and separate the layers, wash the dichloromethane layer with 500ml of 5% hydrochloric acid, combine the water layers, and add 5 mol to the water layer /L sodium hydroxide to adjust the pH to 10, reflux the reaction at 80°C for 8 hours, cool to room temperature, extract with dichloromethane, dry and concentrate to obtain N-(2,6-dimethylphenyl)-2-(1- Piperazinyl)acetamide.
Main reference materials
[1] CN201410539916.4 A kind of quaternary ammonium salt compound and its application
[2] CN201811270825.X Preparation method of ranolazine

 微信扫一扫打赏
微信扫一扫打赏

