Overview[2]
2-Chlorophenylacetylene is an organic intermediate that can be prepared from 2-bromochlorobenzene in two steps.
Apply[1]
CN201811180665 reports a thermoluminescent switch material with information encryption-decryption function, preparation method and application. The material is composed of platinum (II) and 3-trimethylsilyl ethynyl-1 , a complex formed by 10-phenanthroline ligand and 2-chlorophenylacetylene auxiliary ligand. After the material combines dichloromethane molecules and 1,2-dichloroethane molecules, two samples are obtained that are indistinguishable to the naked eye under sunlight or ultraviolet light. The color is yellow and the emission is green. The sample combined with dichloromethane molecules will turn orange-red when heated at 90°C, while the color and luminescence of the sample combined with dichloroethane will change to orange-red only when heated at a temperature above 102°C. Based on the characteristics of color and luminescence changes of these two samples at different temperatures, they can be used to implement information encryption-decryption technology based on one starting material. In addition, the material has the advantages of high sensitivity, high safety, high stability, low cost, strong plasticity, easy operation, and recyclability.
Preparation[2]
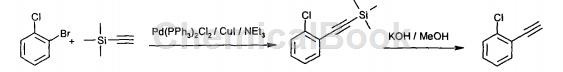
Dissolve 2-bromochlorobenzene (54.1 ml, 0.46 mol) and trimethylsilyl acetylene (72 ml, 0.51 mol) in triethylamine (250 ml). Triphenylphosphine (0.4g, 1.5mmol), copper(I) iodide (0.3g, 1.6mmol) and bis-triphenylphosphine palladium(II) dichloride (0.5g, 0.67mmol) were added and heated under reflux Mix for 18 hours. The reaction mixture was cooled to ambient temperature and 10% hydrochloric acid (480 ml) was carefully added. The product was extracted into hexanes (3×200ml), the extracts were washed with 10% hydrochloric acid (200ml) and water (2×200ml), then dried (MgSO4) and evaporated in vacuo to (2-Chlorophenylethyne)-trimethylsilane (95.4 g) was obtained as an orange oil, which was used without further purification.
At 0°C, add potassium hydroxide (77.5g, 1.38mol) in 4 portions to a mixture of (2-chlorophenylethyne)-trimethylsilane (95.0g, 0.46mol) in methanol (250ml). solution. The mixture was stirred at 0°C until completion of reaction (1:1 ethyl acetate:hexane by tlc). The mixture was neutralized by adding 10% hydrochloric acid and the product extracted into dichloromethane (2 x 150 ml). The combined extracts were dried (MgSO4) and evaporated in vacuo. The remaining oil was purified by short circuit distillation (Kugelrohr) to give 1-chloro-2-ethynylbenzene (41.23 g) as a clear oil. B.pt.38℃/10mbar.250MHz 1H-NMR (CDCl3)δ (ppm): 3.25 (s, 1H) (CH), 7.1- 7.5(m, 4H)(ArH); GC purity 89%, GC retention time 2.67min.
Main reference materials
[1] [Chinese invention] CN201811180665.X A thermoluminescent switch material with information encryption-decryption function, preparation method and application
[2] [Invented in China, authorized by China] CN200780003598.8 7H-pyrido[3,4-D]pyrimidin-8-one, their preparation and application as protein kinase inhibitors

 微信扫一扫打赏
微信扫一扫打赏

