Overview[1]
4-(1H-Benzimidazole-2-yl)phenol is benzimidazole and its derivatives. Benzimidazole and its derivatives are a class of heterocyclic compounds with potential anti-cancer and bactericidal biological activities. It has been widely researched and applied in the pharmaceutical industry, agriculture and other aspects. In addition, benzimidazole has good application value in various fields such as catalysts, new curing agents for epoxy resins, intermediates for organic synthesis reactions, transition metal ligands, and fluorescent probes.
Preparation[1-2]
Method 1, CN201810560072 discloses a method for synthesizing benzimidazole compounds. That is, using substituted o-phenylenediamines and benzaldehyde derivatives as raw materials, using organic protonic acids as catalysts, and using stirring means to efficiently catalyze and quickly prepare benzimidazole compounds. Compared with the methods described in the prior art, this system has clean reaction conditions, simple operation, high yield, safety, low cost and environmental protection.
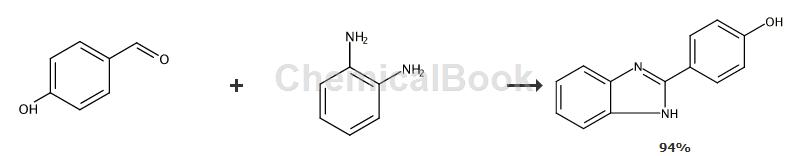
2-(4-Hydroxyphenyl)-1H-benzimidazole: Add 1 mmol o-phenylenediamine and 1 mmol p-hydroxybenzaldehyde to the reaction vessel, then add 0.8 mmol acetic acid, place in a stirrer, 25 Continuously stir for 10 minutes. After the reaction was completed, the product was concentrated under reduced pressure, and the product was purified by column chromatography to obtain a white solid with a yield of 94%.
Method 2,
Zareyee et al. reported that a mixture of benzene-1,2-diamine (1 mmol) and the appropriate aldehyde (1 mmol) in water (5 mL) was stirred at room temperature in a round-bottomed flask. in the presence of SBA-15-Ph-PrSO3H (0.012 g, 2 mol%) or CMK-5-SO3H (0.046 g, 2 mol%) for the appropriate time. When the reaction was complete (TLC; Hexane-EtOAc, 4:1), hot EtOH was added, the mixture was filtered and concentrated in vacuo to give the crude product, which was purified by crystallization (EtOH) to give 2-phenyl-1H-benzene Imidazoles, yield: 89%
Main reference materials
[1] [Chinese invention] CN201810560072.X A method for the synthesis of benzimidazole compounds catalyzed by organic protonic acid under solvent-free conditions
[2]Synlett, 27(8), 1251-1254; 2016

 微信扫一扫打赏
微信扫一扫打赏

