Background and Overview
4-Formylbenzeneboronic acid is a boric acid species that can be used in the Suzuki reaction.
Preparation[1]
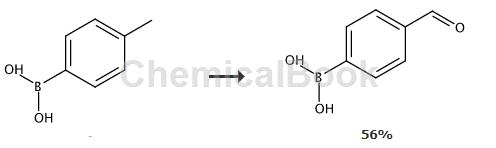
In a 500 ml three-necked flask with mechanical stirring, nitrogen inlet and reflux condenser, add 21.8 g (0.1 mol) p-toluene borate pinacol ester and 35.6 g (0.2 mol) N-bromo Succinimide, 150 ml of dioxane and 0.24 g (0.001 mol) of benzoyl peroxide, then heated and refluxed under nitrogen protection until p-toluene borate pinacol ester basically disappears, then add 150 ml of water , continue to heat and reflux for 12 hours, change to a distillation device, evaporate most of the solvent, cool the residue to room temperature, add methylene chloride for extraction, dry over anhydrous sodium sulfate, filter, add petroleum ether until cloud point appears, lower temperature (0 -5°C) and let stand, filter and precipitate the product to obtain 16.5 g of p-aldehyde phenylboronic acid pinacol ester, with a yield of 71% and a melting point of 54-56°C. 1HNMR (400MHz, CDCl3)δ (ppm): 10.04 (s, 1H, CHO); 7.95 (d, 2H, J =8.2Hz), 7.84(d,2H, J=8.0Hz), 1.35(s,12H,CH3). 13CNMR (100MHz, CDCl3)δ (ppm): 192.70, 138.35, 135.42, 128.89, 84.53, 25.08.
Apply [2]
1. Used in the synthesis of (E)-4”-(2-quinoline-2-vinyl)-biphenyl-4-carbaldehyde
(E)-4′-(2-quinoline-2-vinyl)-biphenyl-4-carbaldehyde contains quinoline, styrene, biphenyl, and benzaldehyde structural units. CN201110119842.5 provides a method for preparing (E)-4′-(2-quinoline-2-vinyl)-biphenyl-4-carbaldehyde, which is based on (E)-2-(4-bromostyrene). )quinoline and 4-formylbenzeneboronic acid are used as raw materials to prepare (E)-4′-(2-quinoline-2-vinyl)-biphenyl-4-carbaldehyde. The reagents used are easy to obtain, the reaction operation is simple, the raw materials are saved, the cost is low, and the efficiency is high.
The method for preparing (E)-4′-(2-quinoline-2-vinyl)-biphenyl-4-carbaldehyde is: using (E)-2-(4-bromostyryl)quinoline Use 4-formylphenylboronic acid as raw material, tetrakis triphenylphosphorus palladium as catalyst, add sodium carbonate and solvent under nitrogen protection, heat the mixture to 85°C in an oil bath under magnetic stirring, react for 5 hours, stop the reaction, and cool to room temperature. Add the extractant ethyl acetate and water for extraction, collect the upper organic layer, and extract the aqueous layer twice with ethyl acetate. Combine the upper ethyl acetate layer, dry it with anhydrous sodium sulfate, and evaporate the solvent. The crude product is separated by column chromatography. Purify to obtain a light yellow solid product; the molar ratio of the (E)-2-(4-bromostyryl)quinoline and 4-formylbenzeneboronic acid is 1.0-1.2:1; the catalyst tetrasan Phenylphosphorus palladium is 1% mole of 4-formylphenylboronic acid; the molar ratio of the sodium carbonate and 4-formylphenylboronic acid is 2.0-2.4:1; the solvent is ethylene glycol dimethyl ether, Water and ethanol, glycol dimethyl ether: water: ethanol volume ratio 15:3:5; the extraction agent is ethyl acetate and water, the volume ratio of ethyl acetate and water is 1:1; the described The crude product was separated by column chromatography and the volume ratio of ethyl acetate to petroleum ether was 1:5 as the developing agent. The (E)-4′-(2-quinoline-2-vinyl)-biphenyl-4-carbaldehyde prepared by the invention is a new compound. The reagents used are easy to obtain, the reaction operation is simple, the raw materials are saved, and the cost is low. , with high efficiency and yield of 77~83%, it is a preparation method suitable for industrial production.
2. Used in the synthesis of 4-(E-3-ethoxy-3-oxo-1-propen-1-yl)phenylboronic acid
4-(E-3-ethoxy-3-oxo-1-propen-1-yl)phenylboronic acid is an important intermediate and is widely used in fine chemicals, medicines, pesticides, fuels, and functional Materials and other chemical engineering and chemical pharmaceutical fields. In recent years, 4-(E-3-ethoxy-3-oxo-1-propen-1-yl)phenylboronic acid has been used in the treatment of breast cancer in the degradation of monocyclic or polycyclic female receptors and the synthesis of modulators, etc. Aspects have been used as intermediates.
CN201611153518.4 provides a preparation method of 4-(E-3-ethoxy-3-oxo-1-propen-1-yl)phenylboronic acid. Dissolve lithium chloride and DBU in acetonitrile, adjust the temperature to 0°C, add triethylphosphonoacetate and stir for 9-11min, preferably 10min, raise the temperature to room temperature, stir for 25-35min, preferably 30min, add 4-formyl Phenylboronic acid can be synthesized by reacting at room temperature for 3 to 8 hours. The technical effect of the present invention is reflected in: using 4-formylphenylboronic acid as raw material and obtaining the target product 4-(E-3-ethoxy-3-oxo-1-propen-1-yl through Wittig-Horner reaction )phenylboronic acid, the yield can reach about 80%, and the cheap and easily available organic reagents lithium chloride and DBU are used to avoid low-temperature operation, making the reaction conditions mild, the preparation process simple, and the atom economy of triethylphosphonoacetate Higher efficiency and less pollution. In particular, the invention can realize the purification of the target product by subjecting the target product after the reaction to extraction, reduced pressure concentration and evaporation, acidification, suction filtration, beating and other steps. The subsequent post-treatment process is simple and suitable for industrial application.
3. Can be used to synthesize fluorene derivatives
CN201611088501.5 provides a synthesis method of fluorene ester derivatives with readily available raw materials, simple operation, and mild reaction conditions, overcoming the shortcomings of the existing technology. The synthesis steps are: under argon or nitrogen protection conditions, mix 9, 9-dimethyl-2, 7-dibromofluorene, 4-formylphenylboronic acid and potassium phosphate in a molar ratio of 1: 1.2: 1.5 Dissolve in dioxane, add tetrakis triphenylphosphine palladium catalyst, the dosage of tetrakis triphenylphosphine palladium catalyst is 1~2% of 9, 9-dimethyl-2, 7-dibromofluorene, at 90~ Reflux the reaction in a 120oC oil bath for 15 to 30 hours; after cooling, first extract the system with ethyl acetate and saturated brine with a volume ratio of 1:2, and then wash the organic layer with saturated brine; collect the organic phase and use It is dehydrated with sodium sulfate, filtered, evaporated under reduced pressure, and the solvent is removed; finally, a mixed solvent of petroleum ether and methylene chloride with a volume ratio of 1:1 is used as the eluent and separated by column chromatography to obtain the product Reflux with methanol as the solvent for 1 to 2 hours to finally obtain the compound: 9, 9-dimethyl-2-bromo-7-(4-methoxycarbonylphenyl)fluorene. The new fluorene derivative synthesis method of the present invention has the following advantages compared with the existing technology:
1. Raw materials are easy to obtain, saving costs;
2. Simple operation;
3. The reaction conditions are mild.
Main reference materials
[1] CN201710863987.3 A synthesis method of acyl aryl borate compounds
[2] CN201110119842.5 A preparation method of (E)-4”-(2-quinoline-2-vinyl)-biphenyl-4-carbaldehyde
[3] CN201611153518.4 A preparation method of 4?(E?3?ethoxy?3?oxo?1?propylene?1?yl)phenylboronic acid
[4] CN201611088501.5 New synthesis method of fluorene derivatives

 微信扫一扫打赏
微信扫一扫打赏

