Background
Potassium para-aminobenzoate is an organic synthesis intermediate and pharmaceutical intermediate that can be used in laboratory research and development processes and chemical and pharmaceutical synthesis processes. It is the potassium salt of para-aminobenzoic acid.
Preparation[1]
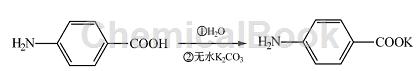
Add 50 mL water and 3g (0. 022 mol) para-aminobenzoic acid into a 100 mL round-bottomed flask, stir well, slowly add 1. 5g (0. 011 mol) anhydrous potassium carbonate, and adjust The pH is about 8. The reaction is stirred for 1 hour, and the impurities are removed by filtration to obtain potassium p-aminobenzoic acid.
Apply[1]
P-Aminobenzoic acid and its N-alkylated derivatives are important optoelectronic functional material compounds. This type of compound can undergo intramolecular electron transfer and even charge separation and intramolecular charge transfer distortion under light induction. Therefore, it is of certain significance in photophysics research and the construction of optoelectronic devices. In addition, this type of compound is also a new pesticide compound that is expected to be used to induce resistance in plant systems. Chen Zhaobin and others have synthesized the existing electron-withdrawing group ( Carboxyl group), and 4-(N-isopropylamino)-benzoic acid with an electron-donating group (N-isopropylamino).
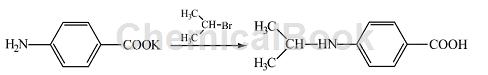
Preparation of 4-(N-isopropylamino)-benzoic acid:
Add 2.5 g (0.02mol) 2-bromopropane to the prepared potassium p-aminobenzoic acid salt solution, and heat and reflux for 12 hours until the 2-bromopropane in the lower layer basically disappears, and a small amount of solid precipitates. After cooling, a large amount of solid precipitated. The solid was filtered out and recrystallized with isopropyl alcohol (added activated carbon for decolorization) to obtain white crystals, which were dried to obtain 1.5 g of the product 4-(N-isopropylamino)-benzoic acid. Rate 51%, m p 160~161°C, IRυ max /cm–1: 3408 (N-H stretching), 3200 (-OH), 2970 (C-H stretching, 1720 (C=O), 1525, 1580, 1600 (benzene ring), 1379, 1384 (bimodal, isopropyl), 837 (para-substituted benzene ring).
Main reference materials
[1] Chen Zhaobin, Feng Liheng. Research on the synthesis of 4-(N-isopropylamino)-benzoic acid [J]. Chemistry World, 2003(11):582-584.

 微信扫一扫打赏
微信扫一扫打赏

