Background and overview[1]
P-methylacetophenone is colorless needle-like crystals or colorless to nearly colorless liquid. This product has an aroma similar to hawthorn flowers, and has a fragrance like alfalfa, honey, and strawberry. The floral and fruity aroma is sharp and sweet. It can be used to prepare acacia-type and lilac-type flavors for soaps, and can also be used as fruit food flavor. It is prepared from the acetylation reaction of toluene and acetic anhydride in the presence of anhydrous aluminum trichloride. Para-methylacetophenone is an important fine chemical intermediate in the field of food and chemical industry. It can be used as a food additive and an intermediate in the production of other high value-added products. Its natural product is found in rosewood essential oil and mimosa flower oil. .
Preparation[1]
CN201610777734.X provides a method for preparing p-methylacetophenone with better performance, more environmental protection and a simple production process. In order to achieve the above object, the technical solution adopted by the present invention is: a preparation method of p-methylacetophenone provided, including the following process steps:
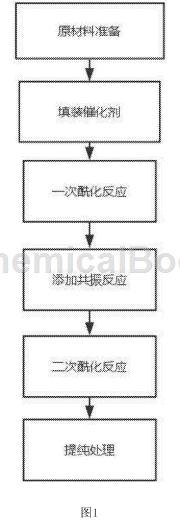
(1) Raw material preparation: The raw materials for the production of methyl acetophenone include the following components (parts by weight): 3-5 parts of toluene, 50-60 parts of acylating reagent, 100-120 parts of organic solvent, 5 parts of catalyst -10 parts, aluminum trichloride 10-15 parts;
(2) Filling the catalyst: Fill the catalyst evenly into the fixed bed reactor, and preheat the fixed bed at a preheating temperature of 120-150°C;
(3) Primary acylation reaction: Feed the toluene and acylating reagent in step (1) into the fixed bed reactor in proportion, and perform pressure heating at a heating temperature of 150-200°C and a reaction time of 20-30min;
(4) Add resonance reaction: Add aluminum trichloride to the solution obtained in step (3) for reaction, the reaction time is 10-20min;
(5) Secondary acylation reaction: Add the organic solvent to the reaction solution obtained in step (4), and increase the heating temperature and pressure. The heating temperature is 250-300°C;
(6) Purification treatment: Pass the mixed solution obtained in step (5) through a distillation tower for continuous rectification, and the distillation temperature is 160-180°C.
The present invention uses toluene and acylating reagent as main raw materials, performs acylation reaction to obtain p-methylacetophenone, and adds an additional acylation reaction process based on the traditional process, including a primary acylation reaction and a secondary acylation reaction. chemical reaction, and obtain p-methylacetophenone with higher purity through a stepwise acylation reaction, and the reactor in this preparation method has a simple structure, stable operation, easy control, and is easy to achieve large-scale and continuous production, and is worthy of promotion. Figure 1 is a flow chart of a preparation method of p-methylacetophenone according to the present invention.
Apply [2-5]
1. Used in the preparation of celecoxib
Celecoxib, trade name: Celebrex, chemical name: 4‑[5‑(4‑methylphenyl)‑3‑(trifluoromethyl)‑1H‑pyrazole‑1‑ [Basic]benzenesulfonamide, with the chemical formula C17H14F3N3O2S, is the first specific type 2 epoxygenase (COX‑2) inhibitor launched by the American Searle Company. Its selectivity for COX‑2 is 375 times that of COX‑1. , mainly used for the treatment of osteoarthritis and rheumatoid arthritis. Its anti-inflammatory activity is equivalent to that of indomethacin, but it has almost no gastrointestinal side effects. The synthesis method of celecoxib mainly involves Claisen condensation of p-methylacetophenone to obtain β-diketone intermediate, and then cyclization with p-hydrazinobenzenesulfonamide to obtain celecoxib.
2. Used in the synthesis of 4-chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H-imidazole-1-sulfonamide
4-Chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H-imidazole-1-sulfonamide (cyanofenazole) is manufactured by Ishihara Sangyo Co., Ltd. The new generation imidazole fungicide developed by the company and jointly with BASF is mainly used to prevent and control diseases caused by Oomycete pathogens, such as potato late blight, tomato late blight, grape downy mildew, cucumber downy mildew, etc. It has good protective activity, long residual effect, and is resistant to rain erosion. It can be used for soil treatment or foliar spraying, and has very low toxicity and good environmental compatibility.
CN201410165615.X provides a method with short reaction cycle and simple post-processing, suitable for batch industrial production of 4-chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H -Method of imidazole-1-sulfonamide. The synthetic route provided by the present invention is as follows:
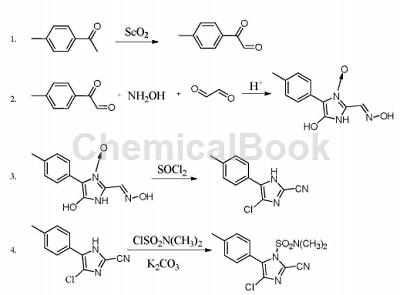
In the first step, 2-carbonyl-2-p-benzylacetaldehyde is synthesized from p-methylacetophenone. The operation is as follows: add selenium dioxide, water and dioxane into three ports Reaction bottle, heat to 50-70°C to dissolve selenium dioxide, add p-methylacetophenone, heat to 90-120°C, react for 4-6 hours, cool to room temperature, filter out the generated elemental selenium, and reduce the pressure Concentrate and remove the solvent, add petroleum ether for pulping, and obtain crude 2-carbonyl-2-p-benzylacetaldehyde. Use a mixed solvent of petroleum ether and ethyl acetate for recrystallization to obtain 2-carbonyl-2-p-benzylacetaldehyde with a purity of more than 98%. 2-p-Benzylacetaldehyde. Among them, the molar ratio of p-methylacetophenone, selenium dioxide, water and dioxane is 1:1~2:0.1~0.5:3~10, and the volume ratio of petroleum ether and ethyl acetate is 10~15 :1.
3. Used in the synthesis of 4-acetylbenzoic acid
4-Acetyl benzoic acid mainly synthesizes the new drug intermediate N-methyl-2-(4-acetyl-5-nitrobenzimidazole, benzimidazole and its Derivatives are the active ingredients of many new drugs. For example, clominazole antihistamines, ethinazine, strong analgesics, benzylchloride plus imidazole, antispasmodics and antifungal drugs belong to this type of derivatives, and their synthesis has certain Theoretical significance and strong practical value. In the past, the production cost of synthesizing 4-acetyl benzoic acid was high, and the three waste emissions were large, which greatly affected the use and promotion. In the existing technology, the reaction temperature is high, the dosage of potassium permanganate is large, and the reaction time is long .CN201210161777.7 provides a preparation method of 4-acetyl benzoic acid with low production cost, environmentally friendly production process and high yield. It includes the following steps:
(1) Oxidation, add p-methylacetophenone, water and anhydrous zinc chloride into the reaction pot, stir evenly, slowly raise the temperature to 35~40℃, divide the potassium permanganate into five equal parts, each Add one portion in 15 to 20 minutes, and control the reaction temperature to 48 to 55°C. After the addition, control the reaction temperature to 40 to 45°C, keep it warm for 1.5 hours, then lower the temperature to 17 to 22°C, centrifuge, and dry to get 4- Crude Acetyl Benzoic Acid.
(2) Mix the prepared crude 4-acetyl benzoic acid with anhydrous acetic acid, heat and reflux for 0.5 to 1.5 hours, filter while hot, centrifuge and dry to obtain 4-acetyl benzoic acid.
Main reference materials
[1] Concise Dictionary of Fine Chemicals
[2] CN201610777734.X Preparation method of p-methylacetophenone
[3] CN201210027318.X New method for preparing celecoxib
[4] CN201410165615.X A synthesis method of 4-chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H-imidazole-1-sulfonamide
[5]CN201210161777.7 A preparation method of 4-acetyl benzoic acid

 微信扫一扫打赏
微信扫一扫打赏

