Background and overview[1][2]
2,4-Dichlorophenylacetic acid is a key intermediate for the new insecticide and acaricide spirodiclofen. Spirobifen is a kind of insecticide and acaricide developed by the company, such as oxobutyrolactone with a cyclic ketone structure. It is an active ingredient of “mite hazard”. Spirobifen has the same Existing acaricides have a completely different mechanism of action. It destroys the energy metabolic activities of mites by inhibiting fat synthesis in the body of the mites, and ultimately kills the mites. Therefore, there is no cross-resistance problem with existing acaricides. Hangzhou Scientific Company introduced 24% mite hazard suspension into the Chinese market in 2005, which has good business prospects. At present, there are no reports on the synthesis process of 2,4-dichlorophenylacetic acid in China. Therefore, exploring an economical and reasonable synthesis process route of 2,4-dichlorophenylacetic acid is of great significance to the industrial production of spirodiclofen in China. Literature reports that this compound mainly has the following synthesis route: benzyl chloride catalyzed carbonylation, using 2,4-dichlorobenzyl chloride as raw material, and palladium chloride catalyzed carbonylation under high temperature and high pressure conditions to synthesize 2,4-dichlorophenylacetic acid in one step . This synthesis method is green and environmentally friendly, but the reaction needs to be completed under high temperature and high pressure conditions, which requires high equipment and equipment, and is difficult to operate. It uses expensive palladium chloride as a catalyst, which is difficult to recover and difficult to achieve industrial production
Apply[2-4]
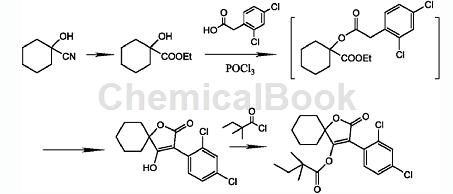
2,4-Dichlorophenylacetic acid is a key intermediate for the new insecticide and acaricide spirodiclofen. Spirodiclofen is a low-toxic miticide with a new structure developed by the company. It has contact killing and stomach poisoning effects, but has no systemic activity. It has a broad spectrum of killing mites, long lasting effect, and kills both eggs and young mites. Spirobifen mainly inhibits the fat synthesis of mites and blocks the energy metabolism of mites. It is effective for all developmental stages of mites. It has outstanding ovicidal activity and has no cross-resistance with existing acaricides. It is suitable for use in Control harmful mites that are resistant to existing acaricides. There is research and development of a preparation method for spirodiclofen and its intermediates. 3-(2,4-Dichlorophenyl)-2-oxygen is prepared in one step using methyl hydroxycyclohexylcarboxylate, 2,4-dichlorophenylacetic acid, and the action of phosphorus oxychloride or phosphorus trichloride. Generation-1-oxaspiro[4,5]dec-3-en-4-ol is finally reacted with 2,2-dimethylbutanoyl chloride to obtain spirodiclofen. This process has short reaction time, low reaction temperature, and the yield can be increased by more than 10% (based on 2,4-dichlorophenylacetic acid). This route is suitable for industrialization. Its synthesis route is as follows:
In addition, 2,4-dichlorophenylacetic acid can also be used to prepare a black wolfberry culture medium, including macroelement components, trace element components, iron salt components, organic matter components, plant hormone components, muscle alcohol, agar powder and sucrose; the macroelement components include: potassium nitrate, ammonium nitrate, calcium chloride dihydrate, magnesium sulfate heptahydrate and potassium dihydrogen phosphate; the trace element components include: potassium iodide, boric acid, sulfuric acid Manganese, zinc sulfate heptahydrate, sodium molybdate, copper sulfate pentahydrate and cobalt chloride; the iron salt components include: ferrous sulfate and disodium ethylenediaminetetraacetate; the organic components include: nicotinic acid, Pyridoxine hydrochloride, thiamine hydrochloride and glycine; the plant hormone components include: 2,4-dichlorophenylacetic acid, α-naphthylacetic acid and 6-benzylaminopurine; the culture medium uses an acid-base indicator Adjust pH to 5.8-6.0. The black wolfberry medium provided has a reasonable formula and easily available ingredients, which can allow the stems and leaves of black wolfberry to differentiate in a sterile environment, induce seedlings, and break the problem of low reproduction rate of stems and leaves in a natural environment.
2,4-Dichlorophenylacetic acid can also be used to prepare a tetrafluoroetherazole intermediate. Tetrafluranazole is a second-generation triazole fungicide. It contains fluorine in its molecular structure. Its bactericidal activity ratio is 2 to 3 times that of the first generation. It has a broad bactericidal spectrum, high efficiency, and a long-lasting effect of 4 to 6 weeks. It has It has protective and therapeutic effects and has good systemic conduction properties. Suitable crops include cereal crops such as wheat, barley, oats, rye, etc., fruit trees such as bananas, grapes, pears, apples, etc., vegetables such as melons, sugar beets, ornamental plants, etc. It can prevent and control diseases caused by powdery mildew, rust fungus, Beakspora, Sclerotium and Septoria, such as wheat powdery mildew, wheat smut, wheat glimetism, sorghum head smut and Grape powdery mildew, etc. There is research and development of a synthesis method of tetrafluoroetherazole intermediate, using 2,4-dichlorophenylacetic acid as the raw material, including the following steps: Using 2,4-dichlorophenylacetic acid as the raw material, including the following steps: (1) Add the raw materials into a single-neck bottle, then add methanol and concentrated sulfuric acid for reflux reaction, the reaction time is 0.5 to 1 hour, then spin dry, add ethyl acrylate to dissolve, wash, dry, and spin dry to obtain compound 1; (2) Compound 1 1. Mix paraformaldehyde, potassium carbonate and dimethyl sulfoxide and react at 65°C. The reaction time is 1 to 1.5 hours. Cool and pour into water. Then extract with PE, wash with concentrated brine, dry and spin. Compound 2 was obtained dryly. According to the synthesis method of the present invention, the intermediate product can be prepared into the next step intermediate without any treatment, thereby simplifying the process and adapting to industrial production.
Preparation [1]
Using 2,4-dichlorobenzyl chloride as raw material, after cyanidation and alkaline hydrolysis, the intermediate product is directly used to synthesize 2,4-dichlorophenylacetic acid without separation and purification. This route has economical and easy-to-obtain raw materials, simple operation, mild reaction conditions, and is suitable for industrial production.
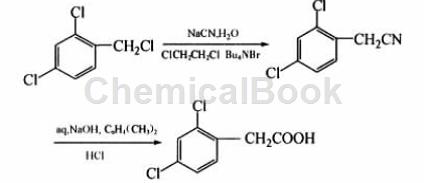
The specific steps are: add 27.1 g (95%, 0.58 mol) sodium cyanide and 100 g water to a 500 mL three-neck flask with a thermometer and mechanical stirring, stir until dissolved, then add 88.5 g (99% , 0.5 mol) 2,4-dichlorobenzyl chloride, 150 mL 1,2-dichloroethane and 1.5 g phase transfer catalyst n-tetrabutylammonium bromide, raise the temperature to 45~55°C, and keep the reaction for 10 hours. After the reaction is completed, add 150 mL of water to the reaction system, separate the layers, extract the water layer twice with 100 mL of 1,2-dichloroethane, combine the oil layers, and evaporate the 1,2-dichloroethane under normal pressure. Cool to room temperature, add 150 mL xylene, 56.5 g sodium hydroxide and 36 g water, heat to 100~110°C, and keep warm for 2 h. After the reaction is complete, cool the reaction solution to 60°C, add 100 mL water and 125 mL (37%, 0.49 mol) concentrated hydrochloric acid with stirring, continue stirring at 5-10°C for 2.5 h, filter with suction, and use 200 mL for the filter cake. Moisture 2 washes. After drying, a white to yellow powdery solid was obtained. After recrystallization with 250 mL of methanol, 87.8 g of white crystal 2,4-dichlorophenylacetic acid was obtained. The liquid chromatography analysis content was more than 99.0%, and the total yield in 2 steps was 84.8%. m.p.128.4 ~129.1℃
Main reference materials
[1] Research on the synthesis process of 2,4-dichlorophenylacetic acid
[2] CN108840846. Preparation method of spirodiclofen and its intermediates
[3] CN108338050. Black wolfberry culture medium and preparation method thereof
[4] CN102993013. Synthesis method of tetrafluoroetherazole intermediate

 微信扫一扫打赏
微信扫一扫打赏

