Background and overview[1]
2′-Hydroxyacetophenone, also known as o-hydroxyacetophenone, is an important pharmaceutical and fine chemical intermediate. For example, it can be used as a raw material for the synthesis of chalcone compounds. It is widely used in the development and industrial production of fine chemical products such as medicines and pesticides. It is a key intermediate for arrhythmia, propafenone and choleretic drugs.
Preparation[1]
There have been relevant reports on the synthesis of o-hydroxyacetophenone. Wang Guoxi (“Journal of Chinese Pharmaceutical Industry”, 1999, 30(5), 232-233) reported that phenol was used as raw material, reacted with acetic anhydride to form ester, and then Fries rearrangement was performed in carbon disulfide solvent to obtain o-hydroxyacetophenone And p-hydroxyacetophenone, the ratio is 2:3. The disadvantage of this method is that carbon disulfide is used as a solvent, which has a pungent smell, is volatile, highly toxic, and inconvenient to handle, and the yield of o-hydroxyacetophenone is only 33%.
In addition, Yang Dingqiao et al. (“Organic Chemistry”, 2002, 22(4), 275-278) reported a solvent-free reaction to synthesize hydroxyacetophenone. Phenyl acetate is heated under the condition of anhydrous aluminum trichloride. Fries rearrangement is performed to obtain ortho-para-hydroxyacetophenone, and the ratio is about 1:1. However, the disadvantage of this method is that the reaction mixture is relatively viscous and difficult to stir, making the reaction difficult to proceed and easy to carbonize. In 2005, Sanjay Bhar et al. (“Synthetic Communications”, 2005, 35(9), 1183-1188) reported that o-hydroxyacetophenone was obtained through hydrolysis reaction using semicarbazone as raw material in the presence of phosphoric acid. This method has expensive raw materials, is difficult to prepare, and is not conducive to large-scale production. In 2007, Ghiaci et al. (“Applied Catalysis A: General”, 2007, 320, 35-42) reported that under the H3PO4/TiO2-ZrO2 catalyst, using phenol as the raw material and ethyl acetate as the acylating reagent, in 200- The gaseous reaction was carried out at 400°C to obtain o-hydroxyacetophenone, with a yield of 96.8%. The catalyst used in this method is difficult to prepare, and the reaction must be carried out at a very high temperature, which is difficult to control and is not suitable for industrial application.
CN200810061619 provides a method for synthesizing o-hydroxyacetophenone that is simple to operate, low in cost, good in safety, high in yield, and easy for industrial production. The method for synthesizing o-hydroxyacetophenone of the present invention includes the following steps:
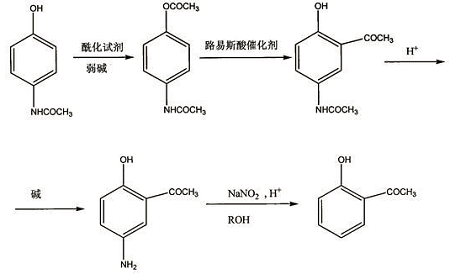
1) Synthesis of 4-acetamidophenol acetate
Dissolve paracetamol in the organic solvent in the reactor, and add a weak base at the same time. The molar ratio of weak base to paracetamol is 1:0.5~3, and then add an acylating reagent. The amount of acylating reagent is the number of moles of paracetamol. 1-6 times; after reacting at 0°C to reflux temperature for 3-36 hours, the organic solvent is evaporated, water is added, and 4-acetamidophenol acetate solid product is obtained by suction filtration;
2) Synthesis of 2-hydroxy-5-acetamidoacetophenone
Dissolve the 4-acetamidophenol acetate in step 1) in an organic solvent in the reactor, and add a Lewis acid catalyst in an amount of 1 mole of 4-acetamidophenol acetate. -10 times, react at 20-180°C for 1-18 hours, cool, filter, and recrystallize to obtain 2-hydroxy-5-acetamidoacetophenone;
3) Synthesis of 2-hydroxy-5-aminoacetophenone
Add 2-hydroxy-5-acetamidoacetophenone and inorganic acid into the reactor in a molar ratio of 1:5~5:1, react at 20°C to reflux temperature for 10-200 minutes, cool, and use Neutralize with a weak base, filter with suction, and recrystallize to obtain 2-hydroxy-5-aminoacetophenone;
4) Synthesis of o-hydroxyacetophenone
Dissolve 2-hydroxy-5-aminoacetophenone in lower alcohol, add copper salt as a catalyst, add concentrated sulfuric acid dropwise at 0-100°C, complete the dropping, stir for 5-60 minutes; then add saturated sulfuric acid dropwise Sodium nitrite aqueous solution, react at 0-100°C for 0.5-5 hours; the molar ratio of copper salt: concentrated sulfuric acid: sodium nitrite: 2-hydroxy-5-aminoacetophenone is (0.005~0.02): (2 ~3):(1~2):1; then perform steam distillation, extract the fraction with an organic solvent, and distill under reduced pressure to obtain o-hydroxyacetophenone.
The reaction equation of the present invention is as follows:
Apply [2]
Pranlukast is a new anti-asthma drug that was launched in the mid-1990s. It is one of the three leukotriene receptor antagonists (LTRAs) that are currently receiving widespread attention internationally. Pranlust has a��Important intermediate: 8-amino-2-tetrazolyl-4-carbonyl-benzopyran, reduced from 8-nitro-2-tetrazolyl-4-carbonyl-benzopyran become.
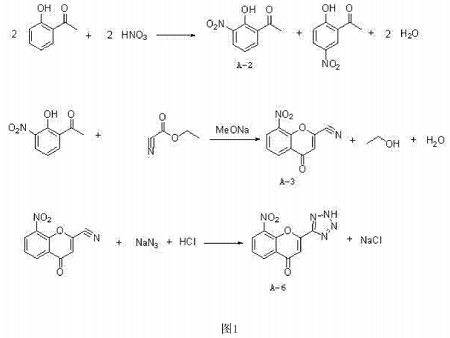
CN201410229357.7 provides a synthetic method for preparing 8-nitro-2-tetrazolyl-4-carbonylbenzopyran from 2′-hydroxyacetophenone, aiming to simplify the operation, reduce costs, and improve The reaction yield is high and it is easy to realize industrial production. The object of the present invention is achieved through the following technical solutions: the synthesis method of 8-nitro-2-tetrazolyl-4-carbonylbenzopyran, using o-hydroxyacetophenone as the starting material, first nitrating with nitric acid , to generate a mixture of 2-hydroxy-3-nitroacetophenone and 2-hydroxy-5-nitroacetophenone, which is then separated and purified by salt formation with an inorganic base, and then hydrolyzed with acid to obtain 2-hydroxy-3-nitroacetophenone. Nitroacetophenone (A-2); 2-hydroxy-3-nitroacetophenone (A-2) undergoes cyclization reaction with ethyl cyanoformate to generate 8-nitro-2-cyano-4 -Carbonylbenzopyran (A-3), finally reacts with sodium azide to form 8-nitro-2-tetrazolyl-4-carbonylbenzopyran (A-6).
Main reference materials
[1] CN94111457.0 A kind of preparation method of o-hydroxyacetophenone
[2]CN201410229357.7 Synthesis method of 8-nitro-2-tetrazolyl-4-carbonylbenzopyran

 微信扫一扫打赏
微信扫一扫打赏

