Overview【1】
Alogliptin benzoate, chemical name is (R)-2-((6-(3-aminopiperidin-1-yl)-3-methyl-2,4-dioxy Generation-3,4-dihydropyrimidine-1(2H)-yl)methyl)benzonitrile benzoate is an inhibitor of serine protease dipeptidyl peptidase IV (DPP-IV) and can maintain glucemia in the body. Glucose-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) levels promote insulin secretion, thus exerting a hypoglycemic effect. Research on diabetes drugs has found that drugs such as alogliptin benzoate have a good curative effect on type 2 diabetes and are well tolerated clinically. Therefore, this type of drug has broad research prospects in the treatment of diabetes and can be foreseen in the future. There will be more alogliptin benzoate drugs coming out.
Synthesis【2】【3】
1. Use 6-chloroouracil (industrial grade) and α-bromo-o-methylbenzonitrile as raw materials, DMF as the solvent, and dimethyl sulfate as the methylating reagent. After 4 steps of reaction, 38.09% The total yield of alogliptin benzoate (5) is obtained, and the reaction conditions are simple.
Synthetic route of alogliptin benzoate:
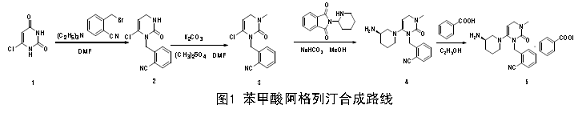
Experimental steps:
(1). Preparation of 2-((6-chloro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)methyl)benzonitrile.
Add 10.0g (68mmol) (1) into a 500ml single-neck round-bottom flask, add 100ml N, N-dimethylformamide, stir and dissolve at room temperature 25°C. After dissolution, slowly add 8.08g (80mmol) triethylamine dropwise under stirring conditions. After the dropwise addition, stir for 0.5h. Dissolve 13.0g (66mmol) 2-cyanobenzyl bromide in 35ml DMF solution and slowly add it dropwise into the reaction. solution and stir the reaction overnight. The DMF in the reaction solution was spin-dried, poured into 8 times the amount of ice water, cooled and crystallized, filtered with suction to obtain a white solid, dried and weighed to obtain 13.86g of the product, with a yield of 79.65%.
(2). 2-[(6-Chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]benzonitrile preparation.
Add 10.0g (38 mmol) (2) into a 500 ml single-neck bottle, then add 140ml DMF solvent, stir and dissolve at room temperature 25°C to obtain a suspension, add 7.6g (55mmol) potassium carbonate and stir for 15 minutes. Dissolve 5.8g (46mmol) dimethyl sulfate in 20ml DMF solution, add it dropwise into the reaction solution using a constant pressure dropping funnel in a closed system, control the dropping speed to 1 to 2 drops/s, and continue stirring the reaction at room temperature after the dripping is completed. 6h. Pour the reaction solution into a 250ml eggplant-shaped bottle and spin dry the DMF solvent in the reaction solution. Pour into 8 times the amount of ice water. Cool and crystallize. Filter to obtain a white solid. After drying, weigh the product to obtain 8.9g. The yield is 84.47%. .
(3). Preparation of alogliptin (4).
Dissolve 3.3g (12mmol) (3) in 50ml dry methanol, stir at room temperature 25°C for 15 minutes to obtain a suspension, and slowly heat up to 100°C. Add 5.0gNaHCO3 (60mmol) and continue stirring for 30min. Add 3.3g (14.4mmol) 3-piperidyl phthalimide and stir for about 20 minutes to dissolve the solid. The solution turns light yellow. Continue the reflux reaction at 100°C for 2 hours. Filter the reaction solution through a sand core funnel and pour it into a 250ml eggplant shape. Spin the reaction solution in the bottle to dryness to obtain a brown solid. Dissolve the solid with dichloromethane. Extract the combined organic layers with water and saturated brine respectively. Spin the organic layer to dryness to obtain 2.85g of light yellow powdery product with a yield of 70%. The crude product is recrystallized from ethanol to obtain
White solid.
(4). Preparation of alogliptin benzoate (5).
Add 1.5g (4.425mmol) (4) and 80 ml ethanol into a 250 ml single-neck bottle and stir at room temperature
Dissolve, control the temperature to 70°C (slowly raise the temperature), add 0.65g (5.31 mmol) benzoic acid and reflux for 2 hours. Cool the solution to 0 ~ 5°C and stir for 12 hours or freeze overnight for crystallization, filter and use for crystallization. After washing with ethanol and drying, 1.65g of white solid was obtained, with a yield of 80.89%.
2. Using o-bromotoluene as raw material, alogliptin benzoate is prepared through 5 steps of adding cyano group to non-toxic potassium ferrocyanide, free radical substitution, alkylation, and substitution reaction.
Synthetic route:
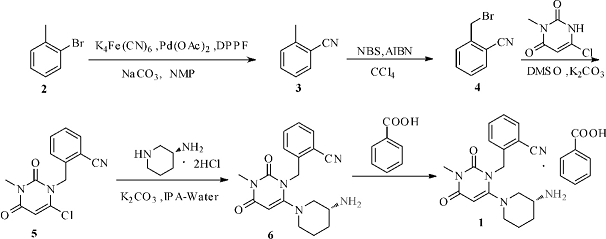
Synthesis experiment:
(1). Synthesis of o-methylbenzonitrile (3)
Under N2 protection conditions, place 9.5g of potassium ferrocyanide (25.8mmol) and 3g of sodium carbonate (1equiv) in a three-necked flask, add 20mL of N-methylpyrrolidone (NMP), and then add Slowly add 5g of o-bromotoluene (29.2mmol) dropwise into the reaction solution, stir at 50°C for 10 minutes, and then add palladium acetate (Pd(OAc)
2) 2. 5mg (0. 05mol%) and 1,1′-bis(diphenylphosphine) ferrocene (DPPF) 5mg (0. 1mol%), stir, quickly heat to 125°C, overnight , follow TLC until the reaction is almost complete, cool, add 30mL of water to dilute, extract with n-hexane (30mL×3), wash with saturated brine (30mL×3), dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain light yellow or colorless 3g of oily liquid was purified to obtain compound 3, 2.7g (yield 80%).
(2). Synthesis of 2-bromomethylbenzonitrile (4)
Add 2.7g (23.05mmol) of compound 3, 4.51g of N-bromosuccinimide (NBS), and 0.3g of azobisisobutyronitrile (AIBN) into the three-necked flask. Under nitrogen protection, add 20 mL of anhydrous carbon tetrachloride solution, raise the temperature to 60°C, follow TLC until the reaction is completed, cool to room temperature, filter with suction, wash the filter cake, and spin dry the solvent under reduced pressure to obtain a light yellow oily irritating liquid 4. It was used directly in the next reaction without further purification.
(3). 2-(6-chloro-3methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile (5) Synthesis
Weigh 4.07g (25.4mmol) of 3-methyl-6-chlorouracil into a three-neck flask, add 20mL of dimethyl sulfoxide (DMSO), stir at 30°C for 20min until the solution is clear, and add carbonic acid Potassium 3.9g, continue stirring for 10min, set aside. Dissolve compound 4 in 5 mL DMSO, slowly add dropwise to the above reaction solution to be used, raise the temperature to 60°C, stir for 2 h, cool, add 30 mL water to dilute, extract with ethyl acetate (20 mL × 3), and wash with saturated brine , dried over anhydrous sodium sulfate, and evaporated the solvent to dryness under reduced pressure to obtain 6.56g of light yellow crude product, which was recrystallized with dichloromethane and n-heptane to obtain 4.45g of white crystal 5 (yield 70%).
(4).(R) -2-[( 6-( 3-aminopiperidin-1-yl) -3-methyl-2,4-dioxo-3,4-dihydropyrimidine- Synthesis of 1(2H)-yl)methyl]benzonitrile (6)
Add 4.45g of compound (5), 3.08g (17.7mmol) of R-3-aminopiperidine dihydrochloride, and 2.94g (1.2equiv) of potassium carbonate into a three-necked flask, add isopropanol and Add 15 mL of water mixed solution, raise the temperature to 65°C and stir overnight. TLC will track the reaction to completion. Add 10 mL of acetonitrile at 65°C, continue stirring for 30 minutes, and cool to
At room temperature, filter and wash the filter cake. The filtrate is spin-dried under reduced pressure to obtain 5.1g of light yellow powder. The crude product is purified to obtain 4.12g of white solid 6 (yield 75%).
(5).Synthesis of alogliptin benzoate(1)
Add 4.12g (12.1mmol) compound 6 and 30mL of ethanol into the three-necked flask, start stirring, raise the temperature to 70°C, insulate for 10 minutes, add 1.5g of benzoic acid, continue stirring for 4h, cool to room temperature, and then Cool in an ice bath for 3 hours, precipitate crystals, filter, and wash to obtain a white crystalline solid 1, 3.91 g (yield 75%).
References
[1] Zhang Shufang, the new drug N E S I N A for the treatment of diabetes has been approved for marketing in Japan, Chinese Practicing Pharmacist, 2010.12, page 54
[2] Zhao Zhe, Ding Huaiwei, Zhao Mingming, Yuan Yue, Meng Ying, Song Hongrui, Research on the synthesis process of alogliptin benzoate, China Medical Guide, 2014.10, page 1353
[3] Jiang Jieying, You Li, Deng Sisi, Deng Junfeng, Liu Yu, Zhang Xiao, Synthesis of alogliptin benzoate, Chemical Research and Application, 2015.12, page 1868

 微信扫一扫打赏
微信扫一扫打赏

