Overview
2,4-Dihydroxyacetophenone is an intermediate in organic synthesis, pharmaceutical synthesis and chemical products. It can be used to prepare the anti-angina drug ethoxyflavone. It can also be used to prepare fine chemical products such as pesticides and flavonoids; it is also an important analytical reagent for determining iron ions.
Synthesis【1】【2】
The preparation of 2,4-dihydroxyacetophenone can be divided into the following routes according to the acylating agent:
(1) Use glacial acetic acid as the acylating agent and zinc chloride as the catalyst; the yield is 61%. Using BF3, H2SO4, polyphosphoric acid and other catalytic systems, the yield can reach 60% to 80%.
(2) Using acetic anhydride as the acylating agent, HClO4 and AlCl3 as the catalyst, the yield is 70% to 90%; using ion exchange resin as the catalyst, the yield is 83.5%.
(3) Using acetyl chloride as the acylating agent and SnCl4 as the catalyst, the yield is 80%; using ion exchange resin as the catalyst, the yield is 52%.
(4) Using acetonitrile as the acylating agent and ZnCl2 as the catalyst, the yield was 77%.
The route using glacial acetic acid as the acylating agent and zinc chloride as the catalyst has readily available raw materials, cheap raw materials, and easy operation. It is a better technical route, but its yield is only 61%. From the perspective of industrial production, how to improve yield and reduce costs is crucial.
1. Use orthogonal design method to optimize the process conditions for preparing 2,4-dihydroxyacetophenone using glacial acetic acid as the acylating agent, hoping to find out the main factors affecting the yield, greatly increase the yield, and reduce cost.
The reaction formula is as follows:
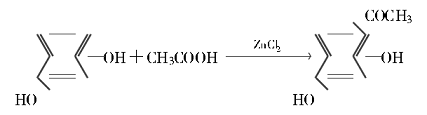
Experimental process: First heat and melt a certain amount of ZnCl2 in a crucible, then add it to a three-necked flask containing a certain amount of glacial acetic acid, and add 11 g (0.1 mol) resorcinol under stirring. The oil bath is heated to a certain temperature. After stirring for a certain period of time, cool. Dilute with 25 mL water and 25 mL concentrated hydrochloric acid, and cool to 5°C in the refrigerator. Filter out the precipitate. Wash 5 times with 120 mL of ice water to remove zinc ions. Boil and dissolve with 180 mL of 1:11 dilute hydrochloric acid. If there is insoluble matter, remove it by filtration, then cool it to 5°C in the refrigerator, filter out the crystals, and dry the product to obtain the product.
The optimal process conditions are: 11 g of resorcinol, 15 g of ZnCl2, 27 mL of glacial acetic acid, reaction time of 5 h, reaction temperature of 140 ℃, yield can reach 91% ~ 93%, product purity ≥ 98%, which is about 30 percentage points higher than the literature value (61%). The raw material cost is reduced by more than 30%. The process is stable and has good application prospects.
2. Under the action of a catalyst, 2,4-dihydroxyacetophenone is synthesized from the raw materials resorcinol and glacial acetic acid through a Fu-G reaction
The classic synthesis method uses glacial acetic acid as the acylating agent and zinc chloride as the catalyst, but it is difficult to decolorize and the yield is not high, mostly 60% to 80%; acetic anhydride is also used as the acylating agent and ion exchange Resin is used as a catalyst; acetyl chloride and acetonitrile are also used as acylating agents, and SnCl4 is used as a catalyst, but these methods are not suitable for industrialization.
Starting from the classic method, we found a method that is both economical and easy to operate, with a yield as high as 97.38%. The synthesis route is as follows:
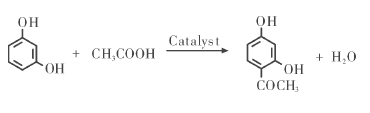
OriginalMaterials: Resorcinol, glacial acetic acid, ethanol, anhydrous methanol, etc. are all of analytical grade.
The optimal reaction conditions are: 12 g of glacial acetic acid, 16 g of catalyst, 1.5 h reaction time, and 135 ℃ reaction temperature; the appearance of the product is nearly colorless needle-like crystals, with a melting point of 144.8 to 145.8 ℃, and infrared The product was identified as 2, 4-dihydroxyacetophenone by UV spectrum and its purity was 96.70%.
3. Using resorcinol and glacial acetic acid as raw materials, 2,4-dihydroxyacetophenone was synthesized with the assistance of microwave
Add 1.10 g (10 mmol) resorcinol, 2.0 ml (15 mmol) glacial acetic acid, 1.25 g (10 mmol) anhydrous zinc chloride into a 100 ml round bottom flask, and irradiate with 150 W microwave for 4 minutes , pour the reaction product into 30 ml of water, an orange precipitate will precipitate, filter under reduced pressure, and wash with deionized water 2 to 3 times to obtain an orange solid. Use 50% ethanol aqueous solution to recrystallize and dry under vacuum to obtain an orange solid. Bulk crystals, yield 67.8%.
The effect of the dosage of acetic acid on the reaction yield: While the dosage of 10 mmol resorcinol and 10 mmol anhydrous zinc chloride remained unchanged, the dosage of glacial acetic acid was changed and 150 W microwave radiation was used for 4 min. It was found that As the dosage of glacial acetic acid increases, the yield of the product increases, and the yield is the highest when the dosage of glacial acetic acid is 15 mmol.
The influence of the dosage of anhydrous zinc chloride on the reaction yield: while the dosage of 10 mmol resorcinol and 15 mmol glacial acetic acid remains unchanged, the dosage of anhydrous zinc chloride is changed, and 150 W microwave radiation is applied for 4 min. , found that as the dosage of zinc chloride increases, the yield of the product gradually increases, and the yield is the highest when the dosage of anhydrous zinc chloride is 10 mmol.
The effect of microwave power on reaction yield: When the dosage of 10 mmol resorcinol, 15 mmol glacial acetic acid, and 10 mmol anhydrous zinc chloride remained unchanged, the microwave power was changed and irradiated for 4 minutes. It was found that with the As the microwave power increases, the yield of the product increases. When the power is 150 W, the yield is the highest. When the power is 200 to 250 W, the yield does not change significantly. At 300 W, carbonization occurs.
The impact of reaction time on reaction yield: Select the best reaction conditions, use TLC to track the reaction, and find that reaction time has a great impact on yield. The yield was highest when the reaction time was 4 minutes, and carbonization occurred when the reaction time was 9 minutes.
References
[1] Lu Yaping, He Jinhuan, Yang Zhongyu, Preparation of intermediate 2,4-dihydroxyacetophenone with high yield, Journal of Zhejiang University of Technology, 2002.04, page 110
[2]Xu Suoping, Xu Guo, Pei Yuan, Microwave synthesis and crystal structure of 2,4-dihydroxyacetophenone, Journal of Xuzhou Normal University (Natural Science Edition), 2010.03, page 68

 微信扫一扫打赏
微信扫一扫打赏

