Background and overview[1][2]
P-methoxybenzaldehyde (p-methoxybenzaldehyde) is an organic synthesis intermediate. As a mid-flavor spice, it is widely used in the preparation of floral flavors; used in medicine, food and daily chemical industries. Para-methoxybenzaldehyde can also be used as a raw material for the preparation of para-methoxybenzyl alcohol and some sunscreen products.
Apply[2]
P-Methoxybenzaldehyde, also known as anisaldehyde, has a long-lasting hawthorn aroma and an attractive fragrance. It is widely used in the preparation of various flavors such as sweet floral, clove and hawthorn, and is also used in food and sugar. It is a spice with high economic value and is used in the preparation of beverage flavors. It is not only an important intermediate in organic preparation, but also an intermediate in the preparation of pharmaceutical porphyrin photosensitizers, hydroxycarbyl penicillins, etc. Its adduct with sodium bisulfite can be used as a metal brightener under alkaline conditions. In agriculture, p-methoxybenzaldehyde can be used as pesticides and pesticide additives, biological growth inhibitors, etc.
1. Preparation of acetal spices
Acetal spices are high-end new spices developed in the past 10 years. Since this type of compound has a floral and fruity aroma that is better than the parent carbonyl compound, the aroma is soft and moist, and the fragrance lasts long. As long as a small amount is added, the natural feel of the spice can be significantly increased. Some fragrances resemble expensive natural sandalwood and are highly sought after. Compared with other aldehyde fragrances, in addition to having a softer and more elegant aroma, it also has extremely strong diffusion power, good chemical stability, does not decompose or deteriorate in ordinary acid-base media, has rich sources of raw materials, and has a simple manufacturing process. Therefore, it has gradually attracted people’s attention in the spice industry. For example, using p-methoxybenzaldehyde and methylmercaptan as raw materials, under the catalysis of p-toluenesulfonic acid, the thioacetal perfume 2-(dimethylthio)methyl-4- was prepared with a yield of 83.7%. Methoxybenzene.
2. Preparation of drugs and cosmetics
P-Methoxybenzaldehyde is not only an important intermediate in organic preparation, but also a raw material for the preparation of drugs and cosmetics. Para-Methoxybenzaldehyde is generally used in vasodilators, antihistamines and other drugs, such as Use it as an intermediate to manufacture the antimicrobial drug amoxicillin and to prepare porphyrin photosensitizers. p-Methoxybenzaldehyde produced by Japan’s Tanabe Pharmaceutical Co., Ltd. is used to prepare the pharmaceutical product diazepam hydrochloride. It is a vasodilator and is mainly used to improve angina, angina, and other conditions, and is exported to the United States in large quantities. p-Methoxybenzaldehyde can be used as a raw material for preparing anti-ultraviolet cosmetics and the pharmaceutical choleretic agent Cholevitamin. Some data show that p-methoxybenzaldehyde has a significant inhibitory effect on Candida albicans nucleic acid metabolism, so it can be used as an additive for the treatment of some gynecological diseases. Preparation of p-methoxybenzaldehyde anthranilic acid Schiff base and its transition metal complexes. The research on such complexes in medicine has attracted increasing attention.
3. Preparation of functional polymer materials
Ferocene polymer is a type of metal-organic polymer with great development and application prospects. After polycondensation, copolymerization and modification, functional polymer materials with special electrical, magnetic and other properties can be obtained. Polyferrocene-p-methoxybenzaldehyde is an important ferrocene condensation polymer.
4. Preparation of aromatic alcohols, ketones, acids and esters
Using natural p-methoxybenzaldehyde as raw material, condensation with natural acetone and hydrogenation to produce natural anise acetone. Anise acetone is not only a food spice, but also a pharmaceutical intermediate. It is the main raw material for preparing the β-receptor agonist drug dobutamine, and natural raspberry ketone is obtained by demethylation reaction of anis acetone. Natural p-methoxybenzaldehyde was used as raw material and oxidized with a special agent to prepare high-yield, high-purity natural anisic acid. It was esterified with methanol or ethanol using the usual method to prepare natural methyl anisate and ethyl anisate. ester. Natural p-methoxybenzaldehyde is used as raw material and natural anisyl alcohol is prepared by catalytic hydrogenation. Compared with the general chemical reduction method, it has the advantages of high conversion rate, high yield, and no three wastes. Natural anisyl acetate can be obtained by esterifying anisyl alcohol with natural grade acetic anhydride.
Preparation [1-2]
1. Preparation route using p-methylanisole as raw material
The preparation route using p-methyl anisole as raw material is as follows:
![]()
There are two oxidation methods: chemical oxidation and electrochemical oxidation. Chemical oxidation is divided into gas phase catalytic oxidation and liquid phase catalytic oxidation. The commonly used catalyst components for gas phase catalysis are V2O5 -P2O5 -CuO -K2SO4, which is carried out at 420~530 ℃; the liquid phase catalytic oxidation catalyst is Co (CH3COO)2, Mn (CH3COO)2 or Ce ( CH3COO)3, the yield can reach 60%, while using Co(CH3COO)2-Cr(CH3COO)2-Ce(CH3COO)3 catalyst the yield can be as high as 76%.
Electrochemical oxidation method is widely used in modern industry. It has many advantages. For example, it can reduce the use of a large number of toxic oxidants, such as chromium salts, manganese salts, etc.; it can automatically control the entire oxidation process, which is beneficial to industrial production, etc. . Electrochemical oxidation methods are also divided into two categories: direct electrooxidation methods and indirect electrooxidation methods. At present, domestic and foreign literature reports mostly use the indirect electrooxidation method, using Ce4+/Ce3+ as the redox medium to prepare p-methoxybenzaldehyde, and some have carried out pilot tests and industrial production. Using the indirect electrooxidation method, Ce4+/Ce3+ is selected as the redox medium, and the Ce4+ generated by electrooxidation is used to oxidize p-methyl anisole into the product anisaldehyde. The reaction equation is as follows:
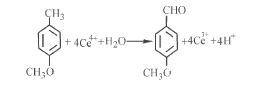
The Ce3+ generated by the reaction is returned to the electrolytic cell for re-electrooxidation. The results show that the Ce4+ electrolysis yield is 83.4%, and the single-pass reaction yield of anisaldehyde is 88.8%. The used media is regenerated and recycled without any three waste emissions. This method has a single reactant, high reaction selectivity, and the electrolyte can be recycled. It has the advantages of simple process, high product purity, one set of equipment can produce a variety of products, and basically no three waste emissions. Therefore, it is easy to implement industrialization and has development prospects. .
2. Preparation route using p-hydroxybenzaldehyde as raw material
This process route is currently used by most countries in industrialization, and the process route is relatively mature. The disadvantage of this method is that the production time is long, but the yield is higher than other methods. This method mainly uses alkylation reaction between p-hydroxybenzaldehyde and dimethyl sulfate under alkaline conditions to prepare the product. The reaction equation is as follows:

At present, there are many research reports on this method. Under the conditions of pH value 8 and reaction 6 h, p-methoxybenzyl
The aldehyde yield is 88%, and the content is not less than 99.3%. On the basis of ensuring that the entire reaction is under alkaline conditions, the sodium hydroxide aqueous solution is added in two steps, and the solvent is added at the same time, and the reaction water is distilled out through azeotropy, and the reaction temperature is carried out in two steps:85~ 95 ℃ is a monomethylation reaction, 135~145 ℃ is a dimethylation reaction, and the reaction time is 7~8 h. The product yield is as high as 93.2%, which provides a better preparation route for the preparation of p-methoxybenzaldehyde.
3. Other preparation routes
The preparation methods of p-methoxybenzaldehyde include p-methoxybenzoic acid reduction method, p-nitrotoluene preparation method, toluene method, p-methoxybromobenzene method, p-methoxybenzonitrile method, and benzene method. Methyl ether method, etc. Among these methods, some raw materials are rare, some catalysts are expensive, some conversion rates are not high, and some production routes are too long or have harsh conditions. Currently, there are only small-scale reports and it is unlikely to be put into industrial production.
Main reference materials
[1] CN201310401210.7 A preparation method of p-methoxybenzaldehyde
[2] Wang Qingjun, Liu Fusheng, Yu Shitao. Research on the preparation and application of p-methoxybenzaldehyde[J]. Progress in Fine Petrochemicals, 2006, 7(5): 34-38.
� Put into industrial production.
Main reference materials
[1] CN201310401210.7 A preparation method of p-methoxybenzaldehyde
[2] Wang Qingjun, Liu Fusheng, Yu Shitao. Research on the preparation and application of p-methoxybenzaldehyde[J]. Progress in Fine Petrochemicals, 2006, 7(5): 34-38.

 微信扫一扫打赏
微信扫一扫打赏

