Background [1]
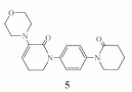
5,6-Dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidinyl)phenyl]-2(1H)-pyridone was prepared Intermediates of apixaban. Apixaban (1), chemical name is 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidine- 1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c] pyridine-3- Formamide is an anticoagulant drug jointly developed by Bristol-Myers Squibb and Pfizer. It directly acts on coagulation factor Xa. It was approved for marketing by the EU and FDA in May 2011 and December 2012 respectively. It is launched in China under the trade name Aletux and is used to treat venous thrombosis diseases including deep vein thrombosis and pulmonary embolism. The synthesis method of 1 has been reported in many literatures.
Preparation of 1-(4-methoxyphenyl)-6-(4-aminophenyl)-7-oxo-4,5,6,7-tetrahydro-1H-pyrazole [3,4-c] pyridine-3-carboxylic acid ethyl ester is reduced with iron powder, a large amount of iron sludge will be produced, causing serious pollution and is not suitable for large-scale production; Preparation 1 hour , using ammonia water to perform ammonolysis in an autoclave, the conditions are relatively harsh, the reaction time is long, and the reaction is incomplete. Some studies have referred to relevant literature and determined the following route to synthesize 1: 2-piperidone is used as raw material, and 3,3-dichloropiperidin-2-one (2) is obtained by dichloro substitution, and 2 is condensed with excess morpholine to obtain the key Intermediate 3-(4-morpholinyl)-5,6-dihydro-1H-pyridin-2-one (3), 3 and 1-(4-iodophenyl) – 2-Piperidone (4) undergoes nucleophilic substitution to obtain 5,6-dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidinyl)phenyl] -2(1H)-pyridone (5), 1 was obtained through 1,3 dipolar cycloaddition and aminolysis, with a purity of 99.8% and a total yield of 30.2% (based on 2-piperidone). The route process is stable, the reaction conditions are mild, the operation is simple, and it is suitable for industrial production.
Apply[1]
5,6-Dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidyl)phenyl]-2(1H)-pyridone is mainly used To prepare apixaban, the specific steps are as follows:
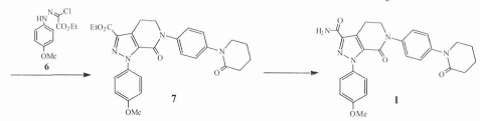
Step 1:1-(4- Methoxyphenyl)-6-[4-(2-oxopiperidin-1-yl)-phenyl]-7-oxo Generation-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl ester (7) Synthesis
At room temperature, 5,6-dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidyl)phenyl]-2(1H)-pyridine Ketone (5.0 g, 14 mmol) and 6 (4.0 g, 16 mmol) were added to ethyl acetate (150 ml), and then potassium iodide (0.35 g, 2 mmol) and triethylamine ( 6.8 ml, 49 mmol), heated to reflux for 12 h, cooled to 0 ℃, added dropwise 4 mol/L hydrochloric acid (20 ml), stirred at room temperature for 2 h, an off-white solid precipitated, filtered. The filter cake was washed with glacial ethyl acetate (20 ml), and the solid obtained was beaten with water (20 ml), filtered, and air-dried at 50 ℃ for 5 h to obtain crude product 7 (6.5 g), which was washed with ethyl acetate. The ester was recrystallized to obtain a white solid 7 (5.8 g, 89%), mp 126 ~ 128 ℃ (Literature 126 ~ 128 ℃), Purity 99.8% (HPLC normalized method).
Step 2: Synthesis of apixaban (1)
0 ℃ Dissolve 7 (5.0 g, 10 mmol) in anhydrous DMF (100 ml), add formamide (4.0 ml, 100 mmol) and Add sodium methoxide (0.83 g, 13 mmol) to the above solution, raise the temperature to 40 ℃ and react for 4 hours. Cool the reaction solution to room temperature and pour it into water (100 ml). A light brown solid will precipitate and stir. 1 h, suction filtration, and drying the filter cake to obtain 1 crude product (4.1 g). Add 1 crude product (4.0 g) to a mixed solvent of ethanol: water (10: 1, 80 ml) at room temperature. Raise the temperature to reflux, cool the solid to 0 ℃ after it is completely dissolved, let it stand for 2 hours, filter with suction, and blow-dry the filter cake at 50 ℃ for 5 hours to obtain off-white crystals1 (3.6 g, 90%), mp 239.5 ~ 240 ℃, purity 99.8% (HPLC normalization method).
Preparation [1]
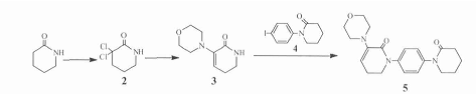
Step 1: Synthesis of 3,3-dichloropiperidin-2-one (2)
In ice bath, slowly drop a solution of 2-piperidone (20.0 g, 202 mmol) in chloroform (200 ml) into a suspension containing phosphorus pentachloride (126.0 g, 606 mmol) in chloroform (300 ml) After adding, slowly raise the temperature to reflux, react for 6 hours, evaporate the solvent under reduced pressure, slowly pour the concentrate into crushed ice (400 g) while stirring, extract with chloroform (400 ml), and use saturated chlorine to separate the organic layer. Wash with sodium solution (200 ml×2) and water (200 ml×2), dry with anhydrous sodium sulfate for 1 h, filter, and concentrate the filtrate to obtain white powder2 (30.5 g, 90.5%). mp162 ~ 165 ℃ ( Document 163 ~ 165 ℃ ).
Step 2: Synthesis of 3-(4-morpholinyl)-5,6-dihydro-1H-pyridin-2-one (3)
At room temperature, add 2 (20.0 g, 120 mmol) to morpholine (60 ml), reflux for 3 h, cool to room temperature, distill under reduced pressure, and evaporate the concentrate with dichloromethane. (200 ml), washed with water (100 ml Powder 3 (10.5 g, 48.4%) mp 145 ~ 148 ℃ (document 145 ~ 147 ℃).
Step 3: 5,6-dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidyl)phenyl]-2(1H)-pyridine Synthesis of Ketone(5)
Add 4 (10.0 g, 33 mmol) and 3 (6.8 g, 36 mmol) to DMSO (160 ml), then add CuI (0.64 g, 3.4 mmol) and anhydrous potassium carbonate (13.7 g, 100 mmol) and phenanthroline (0.66 g, 3.6 mmol), react at 110°C for 12 h under nitrogen protection, cool, filter out the insoluble matter, add dichloromethane (300 ml) to dilute, and then use 6% ammonia water (100 ml× 3) Extract with saturated sodium chloride solution (100 ml×3), combine the aqueous phases, extract with dichloromethane (200 ml×3), combine the organic layers, dry with anhydrous sodium sulfate for 1 hour, filter, and concentrate the filtrate. The crude product 5 (11.0 g) was obtained, which was recrystallized with ethanol to obtain off-white needle-like crystals 5 (9.4 g, 86%), mp 204 ~ 207 ℃ (document 205 ~ 207 ℃), purity 99.7% [HPLC normalization method: Chromatographic column Xbridge Shield RP18 column (4.6 mm × 150 mm, 3.5 μm); mobile phase acetonitrile: water (90: 10); flow rate 1.0 ml/min; detection wavelength 280 nm; column temperature 40 °C; injection volume 10 μl.
Main reference materials
[1]Zhang Wei, Wang Xue, Chang Xinglong, et al. Synthesis of anticoagulant drug apixaban[J]. Chinese Journal of Pharmaceutical Industry, 2016, 47(10): 1216-1218 .

 微信扫一扫打赏
微信扫一扫打赏

