Background and overview[1][2]
2-Chloro-4,6-diphenyl-1,3,5-triazine, density.265g/cm3, boiling point 485.1ºC at 760mmHg. 2-Chloro-4,6-diphenyl-1,3,5-triazine is mainly used as a pharmaceutical and chemical intermediate for the synthesis of other substances. If 2-chloro-4,6-diphenyl-1,3,5-triazine is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse thoroughly with soap and water. If you feel discomfort on your skin, seek medical attention; if it comes into contact with your eyes, separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately. Advice to protect rescuers is as follows: Move the patient to a safe place, consult a doctor, and if conditions permit, please show this chemical safety data sheet to the doctor who comes to the scene. If there is a small leak, collect the leaked liquid in a sealable container as much as possible, absorb it with sand, activated carbon or other inert materials, and transfer it to a safe place. Do not flush it into the sewer; if there is a large leak, build a dike or dig a pit. Contain, seal the drainage pipe, cover it with foam to inhibit evaporation, use an explosion-proof pump to transfer it to a tanker or a special collector, and recycle or transport it to a waste treatment site for disposal.
Apply[1-2]
1. Used to prepare benzimidazole compounds and derivatives, and organic electron transport materials. Organic light-emitting diodes (OLEDs) have important applications in new electroluminescent displays and lighting fields. Among them, high-performance organic molecular electron transport materials are key functional materials for OLEDs, assisting the injection of electrons from the cathode to the light-emitting layer. Amorphous organic electron transport materials with both high thermal stability and film morphology stability are crucial for obtaining highly stable organic light-emitting diodes. The design of sub-transmission materials requires appropriate LUMO (conducive to electron injection) and HOMO energy levels, high thermal stability and film morphology stability, good electron transmission performance and film-forming properties.
So far, a large number of organic small molecule electron transport materials have been reported, but organic small molecule electron transport materials with excellent comprehensive properties generally face challenges in synthesis and purification. In order to overcome the shortcomings and shortcomings of the existing technology, there is research to provide a benzimidazole compound that is easy to synthesize and purify, and can be used to prepare organic functional molecular materials with both high thermal stability and film morphology stability, especially for organic electron transport. Material.
The preparation method of benzimidazole derivatives includes the following steps:
(a) Under a catalytic system, combine benzimidazole compound (2-(6-halonaphthyl-2-yl)-1-phenyl-1H-benzimidazole) and bis-pinacolyl diboron Suzuki coupling reaction (Suzuki reaction) is carried out with alkane, and subsequent treatment is performed to obtain a product containing borate ester; the reaction conditions in step (a) are 80 to 90°C for 3 to 4 hours; so The molar ratio of the benzimidazole compound (the ring-closed halogen-containing intermediate product 2-(6-halogen-naphthalene-2-yl)-1-phenyl-1H-benzimidazole) and the bis-pinacol-based diborane is 1: (1.4~1.5); the catalytic system includes a palladium catalyst, and the palladium catalyst is bis(triphenylphosphine)palladium dichloride; the benzimidazole compound (closed-ring halogen-containing intermediate product 2-(6 The molar ratio of -halonaphthalene-2-yl)-1-phenyl-1H-benzimidazole) to the palladium catalyst is 1: (0.03~0.04); the reaction uses an organic solvent as the reaction medium, and the organic solvent is Conventional organic solvent is preferably tetrahydrofuran; the catalytic system also includes a basic compound, and the basic compound is preferably potassium acetate;
(b) In the catalytic system, combine the borate-containing product of step (a) with 3-bromo-1,10-phenanthroline or 2-chloro-4,6-diphenyl-1 respectively , 3,5-triazine undergoes a coupling reaction and subsequent processing to obtain two structures of benzimidazole compound derivatives. The molar ratio of the borate-containing product to 3-bromo-1,10-phenanthroline or 2-chloro-4,6-diphenyl-1,3,5-triazine is 1: (1~ 1.2).
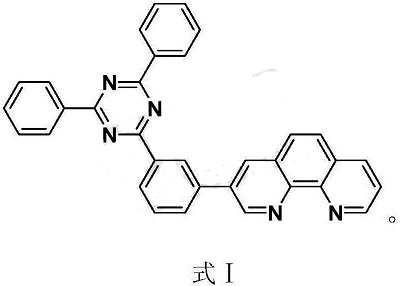
2. Used to prepare organic small molecule electron transport materials, whose structure is formula I.
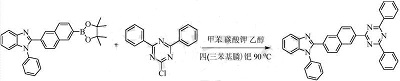
The preparation method is:
(1) Perform a coupling reaction between 2-chloro-4,6-diphenyl-1,3,5-triazine and 3-bromo-phenylboronic acid, followed by subsequent treatment to obtain a bromine-containing intermediate;
p>
(2) Suzuki reaction of the bromine-containing intermediate with pinacol diboron ester, followed by subsequent treatment to obtain the borate ester intermediate;
(3) Perform a coupling reaction between the borate ester intermediate and 3-bromo-1,10-phenanthroline, and perform subsequent processing to obtain an organic small molecule electron transport material.
The above-mentioned organic small molecule electron transport material has a simple structure and has good thermal stability and morphological stability; the n-doped electron transport layer formed by n-doping is used in organic electroluminescent devices , with high luminous efficiency and high stability.
Main reference materials
[1] CN201711228222.9 Benzimidazole compounds and derivatives, organic electron transport materials and their preparation and application
[2] CN201810159745.0 Organic small molecule electron transport materials and preparation, n-doped electron transport layer and application

 微信扫一扫打赏
微信扫一扫打赏

