Background and overview[1][2]
Salicylaldehyde is a fine chemical product with a wide range of uses. Its derivatives are widely used in pesticides, medicines, spices, chelating agents, and dye intermediates, and have a wide range of uses. In medicine, salicylaldehyde can be used to prepare antibacterial drugs. Salicylaldehyde at low concentrations is often used as a preservative in flavors and fragrances due to its strong ability to reduce bacterial activity. In addition, salicylaldehyde is often used as an important ingredient in food, beverages, tobacco, wine, toothpaste, cosmetics, soaps, detergents, etc. At the same time, salicylaldehyde is also an effective tyrosinase inhibitor. After different substituents are introduced into the benzene ring of salicylaldehyde, its biological activities are also different due to different electronic effects and steric effects.
The inhibitory mechanism of 4-methoxysalicylaldehyde on tyrosinase has been studied. Some experiments mainly focused on the inhibitory mechanism of 5-methoxysalicylaldehyde on tyrosinase. The study found that 5-methoxysalicylaldehyde also has the activity of inhibiting tyrosinase, and is similar to 4-methoxysalicylaldehyde. Salicylaldehyde can also inhibit the monophenolase and diphenolase activities of tyrosinase. Both are reversible mixed inhibitors of tyrosinase. It shows that 5-methoxysalicylicaldehyde can act on the free enzyme of tyrosinase or on the complex of tyrosinase and substrate.
Apply[1-5]
5-Methoxysalicylicaldehyde is an important pharmaceutical and chemical intermediate. It can also inhibit the action of tyrosinase. Examples of its applications are as follows:
1. Inhibitory effect of 5-methoxysalicylicaldehyde on mushroom tyrosinase
The IC50 of 5-methoxysalicylaldehyde on tyrosinase monophenolase and diphenolase are 0.76 and 2.45 mmol/L respectively, and the inhibitory intensity is not as strong as that of 4-methoxysalicylaldehyde. The results of the study on the inhibition of tyrosinase by benzaldehyde compounds show that when there is an electron-pushing group at the para position of benzaldehyde, the inhibitory intensity of tyrosinase will be increased. It can be speculated that the inhibitory intensity of 5-methoxysalicylicaldehyde is not as good as that of 4-methoxysalicylicaldehyde because the methoxy group on the benzene ring of 5-methoxysalicylicaldehyde is between the aldehyde groups. position, and the methoxy group of the phenyl ring of 4-methoxysalicylicaldehyde is in the para position of the aldehyde group.
It shows that in benzaldehyde compounds, the type of substituent group on the benzene ring and its position relative to the aldehyde group can affect the effect of the substance on tyrosinase. In addition, 5-methoxysalicylicaldehyde can not only inhibit the steady-state activity of tyrosinase monophenolase, but also prolong the unique lag time of tyrosinase monophenolase. However, 4-methoxysalicylicaldehyde does not affect the lag time of tyrosinase monophenolase. It shows that due to the different substitution positions of the methoxy group, the electronic and steric effects on the benzene ring of 5-methoxysalicylicaldehyde have changed, making its effect different from that of 4-methoxysalicylicaldehyde.
It is further explained that the type and position of the substituent groups on the benzene ring in compounds with benzene rings are crucial to their effects on tyrosinase. By studying the mechanism of 5-methoxysalicylicaldehyde inhibiting tyrosinase, the structure of benzaldehyde compounds was further clarified.
2. Prepare a non-ferrous metal anti-corrosion treatment agent,
The ingredients and mass parts of the treatment agent are: 5-8 parts of salicyl, 3-5 parts of benzoic acid, 5-8 parts of silicone oil, 3-4 parts of phenolic resin, and 2-3 parts of polyimide , 6-8 parts of silicone, 2-4 parts of glycerin, 1-3 parts of sodium pyrophosphate, 3-5 parts of acetic acid, 3-8 parts of oil-soluble phenolic resin, 5-8 parts of 5-methoxysalicylicaldehyde parts and 1-1.5 parts of cyproconazole. The above-mentioned treatment agent overcomes the shortcomings of the existing technology, can effectively prevent metal corrosion and improve the anti-corrosion performance of metal, has minimal environmental pollution, will not harm human health during use, is low in cost, and is easy to promote.
3. Prepare a corrosion inhibitor for metal parts,
The ingredients and mass parts of the treatment agent are: 5-8 parts of salicyl, 3-5 parts of benzoic acid, 5-8 parts of silicone oil, 5-8 parts of 5-methoxysalicylicaldehyde, polyamide 2-3 parts of imine, 6-8 parts of silicone, 2-4 parts of glycerin, 1-3 parts of sodium pyrophosphate, 3-8 parts of oil-soluble phenolic resin, 5-8 parts of 5-nitrosalicylic acid and 1-1.5 parts of cyproconazole. The above-mentioned treatment agent overcomes the shortcomings of the existing technology, can effectively prevent metal corrosion and improve the anti-corrosion performance of metal, has minimal environmental pollution, will not harm human health during use, is low in cost, and is easy to promote.
4. Synthesis of new calix[4]Schiff bases.
Schiff bases are widely used as optoelectronic devices, high-density information storage, optical (or electrical) driven information display devices, light control devices and memory materials. Almost all Schiff bases and their derivatives have photochromic or thermochromic properties. The color-changing mechanism is that in solution, solid state or crystal state, light or heating causes the proton on the hydroxyl group to transfer to the nitrogen atom of the imine. , changes in the molecular structure lead to changes in the absorption spectrum, and this change is reversible.
Studies on the crystal structures of photochromic and thermochromic Schiff base derivatives have shown that the packing of Schiff base molecules in the crystal lattice is very different, and that π-π stacking has an inhibitory effect on proton transfer. The synthesis method is as follows: 2-hydroxy-5-nitrobenzaldehyde (70 mg, 0.44 mmol) or salicylaldehyde (55 mg, 0.44 mmol) or 2-hydroxy-5-methoxybenzaldehyde (66.9 mg, 0.44 mmol) was added to a solution containing 0.22 mmol compound 3 in ethanol (15 mL), and heated to reflux for 8 h. After cooling, a solid precipitated, and the crude product was purified by recrystallization from ethanol.
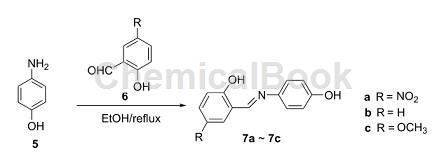
5. Synthesis of 2,5-dimethoxybenzaldehyde.
2,5-Dimethoxybenzaldehyde is an important pharmaceutical intermediate that is difficult to synthesize. It is prepared from p-methoxyphenol as raw material through Reimer-Tiemann reaction catalyzed by polyethylene glycol 10 000 and methoxylation. By comparing the effects of different phase transfer catalysts on the Reimer_Tiemann reaction yield, the best polyethylene glycol-10 000 (PEG-10 000) was selected as the phase transfer catalyst for this reaction. The methoxylation of 2-hydroxy-5-methoxybenzaldehyde is carried out in a buffer solution, and the yield and quality of the product are significantly improved.
The specific method is as follows. In a 250 mL flask equipped with a stirrer, thermometer, and dropping funnel, add 14.0 g of the prepared 2-hydroxy-5-methoxybenzaldehyde, 14. 5 mL water, 30 g borax and part of 10% NaOH solution, stir evenly to make the pH = 9~10, raise the temperature to 60~65°C, maintain it for 30 minutes to completely dissolve, and then cool down. Slowly add dimethyl sulfate dropwise at 45~50°C, measure the pH after a period of time, and strictly control the pH =9~10 by adding dropwise the remaining NaOH. The addition is completed in about half an hour. After the addition, the pH value is measured again at 9~10 and remains unchanged. React for 2.5 hours. After the reaction is completed, cool, filter, and wash until neutral. 14.5 g of the product was obtained by distillation under reduced pressure under nitrogen protection, with a yield of 94% and a melting point of 43~45.
Preparation
In a 250 ml round-bottomed flask equipped with a stirrer, condenser tube, separatory funnel and thermometer, add 320 g powdered sodium hydroxide and 40 mL ethanol. After the temperature drops slightly, add 12.5 g p-hydroxybenzene Methyl ether, 2 mL phase transfer catalyst, dropwise add 16 mL chloroform at 70 ℃, it takes about 3.5~4 h. After the dropwise addition, react for 0.5 h. After the mixture is cooled, transfer it to a round-bottomed flask, add 40~50 mL hot water, cool, and acidify to neutrality with 150~200 mL, 5 mol L H2SO4. Distill the product with water vapor. After the organic solvent is evaporated, collect 500~600 mL of the distilled product, extract the aldehyde with ether, dry the ether with Na2SO4, then evaporate the ether, and collect under N2 protection with a temperature of 133°C under 2 kPa pressure. 2-Hydroxy-5-methoxybenzaldehyde product 10.8 g, yield 72%.
Main reference materials
[1] Inhibitory effect of 5-methoxysalicylicaldehyde on mushroom tyrosinase
[2] CN201510844096.4 A non-ferrous metal anti-corrosion treatment agent and its application
[3] CN201510844065.9 A corrosion inhibitor treatment agent for metal parts and its application
[4] Liu Zhilian, Zhang Shuxiang, Xia Guangming, et al. Synthesis and photochromic properties of new calix[4] Schiff base [J]. Organic Chemistry, 2009, 29(11): 1799-1803.
[5] Chen Zhitao, Xiang Jiannan, Li Zhiliang. A new method for synthesizing 2, 5-dimethoxybenzaldehyde [J]. Journal of Chongqing University: Natural Science Edition, 2002, 25(2): 109-111.

 微信扫一扫打赏
微信扫一扫打赏

