Background and overview[1][2]
3-Chloro-4-hydroxybenzaldehyde can be used as an intermediate for pharmaceutical and chemical synthesis, such as ethyl vanillin, 2-chloro-4-(morpholinylmethyl) phenol and electrochemically active compounds. Ferrocene hydrazone compounds, etc. If 3-chloro-4-hydroxybenzaldehyde is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell; if the eyes are If exposed to sunlight, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately. Advice to protect rescuers is as follows: Move the patient to a safe place, consult a doctor, and if conditions permit, please show this chemical safety data sheet to the doctor who comes to the scene.
Apply[2-3]
3-Chloro-4-hydroxybenzaldehyde is mainly used as an intermediate in organic synthesis. Examples of its application are as follows:
1. Synthesis of 2-chloro-4-(morpholinylmethyl)phenol.
As a heterocyclic structural fragment, morpholine can better adjust the water solubility of the molecule and improve the lipid-water partition coefficient (LogP) of the molecule. Therefore, it is widely incorporated into marketed drugs (such as the antibacterial drug Linel azodone, the antidepressant moclobemide, etc.) and active small molecules. Among the many functionalized structural fragments containing morpholine rings, 4-benzylmorpholine and its analogs have attracted a lot of research interest. Some studies have explored a new synthetic route to prepare the key intermediate 2-chloro-4-(morpholinylmethyl)phenol (1) of this compound. That is, using 3-chloro-4-hydroxybenzaldehyde (2) as raw material, first use allyl bromide to protect the phenolic hydroxyl group to obtain 4-(allyloxy)-3-chlorobenzaldehyde (3), and then use NaBH(OAc )3 reacts with morpholine through reductive amination to prepare 4-(4-(allyloxy)-3-chlorobenzyl)morpholine (4), and finally uses the Pd(PPh3)4/K2CO3 system to remove the alkene The target compound (1) was synthesized using propyl protecting group.
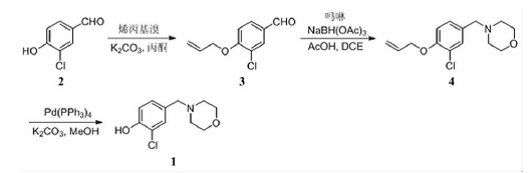
Step 1: Synthesis of 4-(allyloxy)-3-chlorobenzaldehyde (3). In a 100 ml round bottom flask, add 2. 04 g (13 mmol) 3-chloro-4-hydroxybenzaldehyde (2), 1. 69 ml allyl bromide (19. 5 mmol) and 3. 59 g ( 26 mmol) potassium carbonate, dissolved in 60 ml acetone. After filling with argon, the reaction mixture was heated and refluxed for 18 hours, and TLC was used to track and monitor the reaction [v (petroleum ether): v (ethyl acetate) = 3: 1]. After the reaction was completed, it was cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography to obtain 2.0 g of light yellow oil, with a yield of 78%.
Step 2: Synthesis of 4-(4-(allyloxy)-3-chlorobenzyl)morpholine (4). In a 50 ml round bottom flask, add 967 mg (4. 9 mmol) 4-(allyloxy)-3-chlorobenzaldehyde (3) and 0. 43 ml (4. 9mmol) morpholine, followed by 1 . 46 g (6.9 mmol) sodium triacetoxyborohydride, and the reaction mixture was stirred at room temperature overnight under argon protection. After the reaction is completed, add saturated sodium bicarbonate solution, extract with ethyl acetate, dry with anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure. The residue is purified by column chromatography to obtain 895.8 mg of light yellow oil, yield 68%.
Step 3: Synthesis of 2-chloro-4-(morpholinmethyl)phenol (1). In a 25 ml round bottom flask, add 500 mg (1.87mmol) 4-(4-(allyloxy)-3-chlorobenzyl)morpholine (4). After 10 ml of methanol is dissolved, add 21.6 mg (0. 0187 mmol) tetrakis triphenylphosphine palladium, stir at room temperature for 5 min under argon protection, then add 774. 3 mg (5. 6 mmol) potassium carbonate, and continue stirring at room temperature for 3 h. After the reaction is completed, filter, wash the filter cake with methanol, concentrate the filtrate under reduced pressure, add water, carefully adjust the pH value to 7, filter the solid, and recrystallize from n-hexane to obtain 349.1 mg of light yellow solid, with a yield of 82%.
2. Synthesis of electrochemically active ferrocenyl hydrazone compounds.
Ferocene and its derivatives have the characteristics of stability, low toxicity, lipophilicity, rich electricity and redox properties, and can prevent themselves from approaching the active sites of certain enzymes. It also has anti-tumor activity and is therefore often used as a drug to treat certain diseases. Acylhydrazone compounds have both amide and Schiff base functional groups, which can form stable complexes with metal ions in organisms, thereby preventing some enzyme-catalyzed reactions, and have excellent biopharmacological activity. In relation to the biological environmentUnder recent conditions, it can have antibacterial, anti-amoeba, anti-tumor, anti-viral, and anti-tuberculosis effects. The introduction of acylhydrazone groups into ferrocene with redox properties is conducive to the controlled release of drug molecules through external voltage stimulation or potential changes in the physiological environment of diseased tissues and normal tissues. This voltage-sensitive responsiveness is It provides the possibility for active transportation of drugs. Some studies have introduced acylhydrazone functional groups into ferrocene molecules with fat solubility and excellent redox activity, and reacted 1,1′-ferrocene bishydrazide with 3-chloro-4-hydroxybenzaldehyde to synthesize ferrocene. Diacylhydrazone compounds.
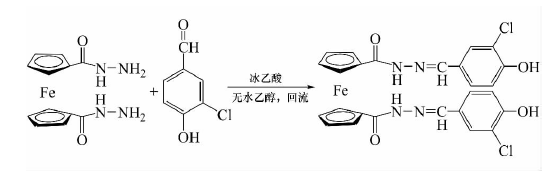
Step 1: Synthesis of ferrocene biscarboxylic hydrazide:. Ferrocene biscarboxylic acid hydrazide was synthesized according to the literature. Under N2 protection, add 15 mL of 85% hydrazine hydrate and 10 mL of absolute ethanol into a 250 mL three-necked flask, stir, reflux and heat, and slowly add 9 g of ferrocene biscarboxylic acid dropwise. methyl ester in 60 mL methanol solution, the reaction slowly appeared bright yellow precipitate, TLC monitoring for about 2 to 4 hours, cooled to room temperature, filtered, washed with absolute ethanol, recrystallized, and dried. m.p.138 ~139℃.
Step 2: Synthesis of ferrocene bisacylhydrazone compounds. Under nitrogen protection, add 0.5 mmol ferrocene biscarboxylic acid hydrazide in 5 mL ethanol solution into a 100 mL three-neck flask, heat to reflux, slowly add 2.5 mL glacial acetic acid dropwise until completely dissolved, and then slowly add 1.1 mmol 3-chloro For a 2 mL ethanol solution of -4-hydroxybenzaldehyde, a large amount of yellow precipitate was formed in about 5 minutes of reaction, and was monitored by TLC for about 2 hours. Cool to room temperature, wash with anhydrous ethanol and petroleum ether in sequence, separate by column chromatography, and dry under vacuum. Orange solid; m.p.257℃; Yield 82%; Purity 97.7%;
3. Preparation of ethyl vanillin. Ethyl vanillin is a broad-spectrum spice and one of the most important synthetic spices in the world today. It is an indispensable and important raw material in the food additive industry. Its aroma is 3-4 times that of vanillin and has a rich aroma. Viburnum bean aroma, and long-lasting fragrance. It is widely used in food, chocolate, ice cream, beverages and daily cosmetics to enhance and fix fragrance. In addition, ethyl vanillin can also be used as a feed additive, a brightener in the electroplating industry, and an intermediate in the pharmaceutical industry. Dissolve 3-chloro-4-hydroxy-benzaldehyde in absolute ethanol, add sodium ethoxide under a nitrogen atmosphere, stir and heat to reflux until the reaction is complete, cool the reaction solution to room temperature, evaporate most of the ethanol under reduced pressure, and add water. Extract the reaction solution with ethyl acetate, combine the ethyl acetate layers, wash with water, dry over anhydrous sodium sulfate and evaporate to dryness under reduced pressure to obtain a white solid, which is recrystallized with cyclohexane to obtain white flake crystals, which are ethyl Vanillin.
Preparation [1]
1) Dissolve p-cresol in methanol, add sodium hydroxide, add rhodium salt and cuprous salt as catalyst, heat to 40~60℃, add oxidant, and react until the reaction is complete , adjust the reaction solution to neutrality, extract the product with an organic solvent and obtain p-hydroxybenzaldehyde through crystallization;
2) Chlorination of p-hydroxybenzaldehyde with chlorine gas to obtain 3-chloro-4-hydroxy-benzaldehyde;
Main reference materials
[1] CN201310408262.7 Synthesis method of ethyl vanillin
[2] Hao Liqiang, Wang Tong, Zhang Ruize, et al. Research on the synthesis of 2-chloro-4-(morpholinylmethyl)phenol[J]. Journal of Taishan Medical College, 2018, 39(2): 121-123 .
[3] Li Yufei, Liu Yajian, Meng Xianjiao, et al. Synthesis, characterization and performance study of electrochemically active ferrocenyl hydrazone compounds [J]. Fine Chemical Intermediates, 2017 (5): 32-34.

 微信扫一扫打赏
微信扫一扫打赏

