Background and overview[1]
Aromatic fluorides have developed rapidly abroad in recent years and are mainly used for the synthesis of physiologically active compounds such as medicines and pesticides. The introduction of fluorine atoms can enhance the efficacy of the medicine and make it sustainable and reduce side effects. Therefore, the research and development of fluorine-containing medicines and pesticides is very active and gradually commercialized. The extensive development and application of fluorine-containing medicines and pesticides have led to a rapid increase in the types of fluorine-containing aromatic compounds. Currently, there are hundreds of types, and the output is also rapid. improve. In recent years, the development, production and export of fluorinated aromatic compounds in my country have also progressed rapidly. Fuxin City is the production base of fluorinated aromatic compounds. In recent years, many products have been developed for domestic and international markets, such as 2-bromo-4-fluorotoluene. This is one example. 2-Bromo-4-fluorotoluene is an intermediate in the synthesis of fluorine-containing pharmaceuticals, and is also mostly used in the synthesis of 2-bromo-4-fluorotoluene acid.
Preparation [1]
Method 1: Synthesis using p-toluidine as raw material, the chemical reaction formula is:
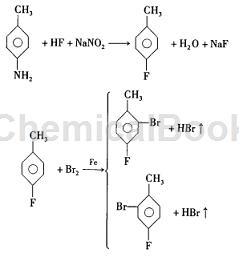
This method uses p-toluidine as raw material, and prepares 2-bromo-4-fluorotoluene through fluorination, bromination, distillation and purification. This route seems simple, but during the experiment it was found that the yield of the product is low, the product is difficult to separate, and the purity of the product is difficult to reach more than 9%, which increases the manufacturing cost of the product and is not conducive to large-scale industrial production.
Method 2: Use p-nitrotoluene as the raw material to synthesize 2-bromo-4-fluorotoluene. This method uses p-nitrotoluene as the raw material and undergoes bromination, reduction, fluorination and distillation to prepare 2- Bromo-4-fluorotoluene. This route seems complicated, but the operation is feasible. Experiments have proved that the raw materials of this synthetic route are easy to obtain, the product purity is high, and the product manufacturing cost is not high, which is conducive to industrial production.
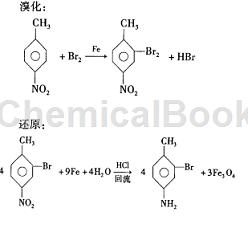
Step 1: Bromination
In a 100 ml three-neck flask, add 2.74g of p-nitrotoluene and 13g of iron powder. Heat and dissolve. Then, under vigorous stirring at 75-80°C, drop in 40g of bromine within 1-1.5 h. After the dropwise addition, the reaction was maintained at 75-80°C for 2 hours, and the yield was 75.05%.
Step 2: Purification
Add 40 ml of glacial acetic acid to the above reactant, heat until the solid is completely dissolved, stir vigorously, and cool to 5°C. Separate the upper layer of glacial acetic acid, add 500 ml of 1% acetic acid solution, heat until completely dissolved, stir vigorously, cool to room temperature, pour off the upper waste liquid, then heat the solid with 500 ml of 1% NaOH, stir, cool and filter out the solid , wash until neutral.
Step 3: Restore
Into a 300 ml three-neck flask, add 150 g H 2 O, 385 g iron powder, 55 g hydrochloric acid, stir vigorously, raise the temperature to reflux, add 350 g crushed 2 in 3-4 times after 30 min. -Bromo-4-nitrotoluene, with intervals of 10 minutes each time, the reflux reaction was maintained for 5 hours, and 2-ol-4-aminotoluene was obtained by steam distillation with a yield of 74.7%.
Step 4: Fluorination
In a 500ml three-neck flask, add 160ml hydrochloric acid, drop 74.5g 2-bromo-4-aminotoluene at 10°C, complete the drop, keep for 5 minutes, cool down below 0°C, add NaNO3 solution (30g NaNO3 + 4 5 9 H2O) after dripping, keep 20min, add 6% HBF4 110g, suction filtration, washing with water and alcohol, drying, pyrolysis, steam distillation, and rectification to obtain 9% of 2-ol-4-fluorotoluene, with a yield of 50%.
Main reference materials
[1] Experimental study on the synthesis of 2-bromo-4-fluorotoluene

 微信扫一扫打赏
微信扫一扫打赏

