Background and overview[1][2]
2-Amino-5-(4-nitrophenyl)-1,3,4-oxadiazole CAS number 51891-79-3, chemical formula C8H6N4O3. Molecular weight 206.15800. Density 1.493g/cm3, boiling point 431.8ºC at 760 mmHg, flash point 214.9ºC, refractive index 1.645. 2-Amino-5-(4-nitrophenyl)-1,3,4-oxadiazole can be used as medicine Chemical synthesis intermediates. If 2-amino-5-(4-nitrophenyl)-1,3,4-oxadiazole is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and wash with soap and water Rinse the skin thoroughly with water and seek medical attention if you feel discomfort. If the eyes come into contact, separate the eyelids, rinse with running water or saline, and seek medical attention immediately. If ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure[1, 3]
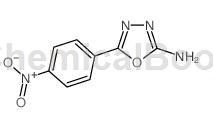
Application [2, 4-5]
2-Amino-5-(4-nitrophenyl)-1,3,4-oxadiazole can be used as a pharmaceutical and chemical synthesis intermediate. Examples of its application are as follows:
1. Prepare a ferrocene-oxadiazolyl-containing Mannich base. The specific steps are as follows: Add 0.8 mmol of 2-amino-5-(4-nitrophenyl)-1,3,4-oxadiazole and 1 mmol of paraldehyde to a dry three-necked flask with a reflux condenser. , and add 30 mL of absolute ethanol as the solvent, add 1.2 mmol of bismuth nitrate pentahydrate as the catalyst during the stirring process, and use a constant pressure titration funnel to dropwise add acetyl ferrocene containing 1 mmol of acetyl ferrocene with a concentration of 0.55 g/mL. Iron in anhydrous ethanol solution, react at room temperature for 4 hours, monitor the reaction progress through TLC plate (indicated when the raw material point of 2-amino-5-(4-nitrophenyl)-1,3,4-oxadiazole disappears The reaction is complete. The developing agent used in the TLC plate is a mixed solution of methylene chloride and methanol with a volume ratio of 10:1). After the reaction is completed, the solvent absolute ethanol is evaporated under reduced pressure by rotary evaporation, and then separated by column chromatography (with A mixture of petroleum ether and ethyl acetate with a volume ratio of 3:1 is used as the first eluent to wash away unreacted acetyl ferrocene, and a mixture of petroleum ether and ethyl acetate with a volume ratio of 2:1 is used as the first eluent. The liquid is the second eluent, collect the second band, and distill the solvent under reduced pressure) to obtain yellow-brown 3-methyl-3-(2-amino-5-p-nitrophenyl-1,3,4 -oxadiazole)-isopropylferrocenylketone, yield 44.1%.
2. Prepare a Schiff base containing carbazolyl and oxadiazolyl groups. The specific steps are as follows: 1) Add 0.005mol 3-acetyl-9-ethylcarbazole and 0.006mol 2-amino-5-(4-nitrophenyl)-1,3,4- to a dry mortar. Oxadiazole and 0.006mol p-toluenesulfonic acid were ground at room temperature for 15 minutes. At this time, TLC monitoring showed 3-acetyl-9-ethylcarbazole and 2-amino-5-p-nitrophenyl-1,3,4 – The raw material point of oxadiazole disappears, indicating that the raw material reacts completely. Then let it stand for 30 minutes to obtain a mixture; the developing agent is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3; 2) Wash the mixture with water and extract After filtration, 3-acetyl-9-ethylcarbazole 2-amino-5-p-nitrophenyl-1,3,4-oxadiazole Schiff base is obtained.
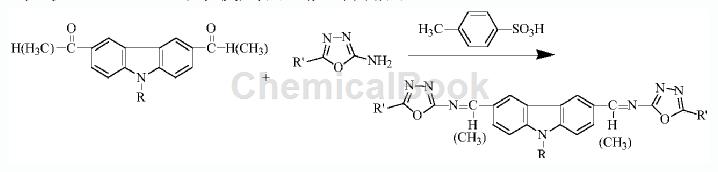
Main reference materials
[1] CN107216357 Preparation method of ferrocenyloxadiazolyl Mannich base
[2] CN106432217 A kind of Schiff base containing carbazole group and oxadiazolyl group and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

