Background and overview[1][2]
2-Bromo-4′-fluoroacetophenone can be used as an intermediate for pharmaceutical and chemical synthesis. If 2-bromomethyl-4-fluorobenzoic acid methyl ester is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse the skin thoroughly with soap and water. If discomfort occurs, Seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Application[1-4]
2-Bromo-4′-fluoroacetophenone can be used as an intermediate for pharmaceutical and chemical synthesis. Examples of its application are as follows:
1. Synthesis of 4′-(N-substituted-1-piperazinyl) chalcone derivatives. Chalcones are a class of compounds with diaryl-substituted α, β-unsaturated ketones as the basic skeleton, and are widely distributed in nature. Chalcones have a unique molecular structure, contain multiple reaction centers, and can be combined with a variety of compounds to exhibit a wide range of biological activities. They are a very important class of intermediates for organic synthesis and drug synthesis. Chalcones extracted and isolated from natural products and synthesized through chemical, biological and other methods exhibit various pharmacological properties such as anti-tumor, anti-parasitic, anti-viral, anti-bacterial, anti-inflammatory, and anti-platelet aggregation. Therefore, the research and development of chalcones has become a hot research field in medicinal chemistry. Using the active substructure splicing method, starting from p-dimethylaminobenzaldehyde and 2-bromo-4′-fluoroacetophenone, the aldol condensation, dehydration, and substitution reactions generate 4-dimethylamino-4′- After (1-piperazinyl)chalcone, chalcone derivatives containing substituted piperazine were designed and synthesized through substitution reaction.
2. Synthesis of nitrogen heterocyclic substituted aminothiazole derivatives. Thiazole rings are an important class of five-membered aromatic heterocycles containing nitrogen and sulfur heteroatoms. They have wide potential application value in many fields such as chemistry, pharmacy, biology, and materials science, and have attracted great attention from many workers [1- 3. Heterocyclic compounds generally have good pharmacological activities, especially nitrogen heterocyclic compounds, which are widely used in the fields of drug synthesis and drug design. By synthesizing compounds, compounds with better pharmacological activity can be obtained. Using 2-bromo-4′-fluoroacetophenone and thiourea as raw materials, 2-amino-4-(4-fluorophenyl)thiazole (1) is obtained through condensation reaction, and then reacts with bromoacetyl bromide to form bromoamide Intermediate (2) was then reacted with imidazole, triazole and benzimidazole respectively to synthesize 5 new N-heterocyclic substituted aminothiazole compounds (3a~3e):
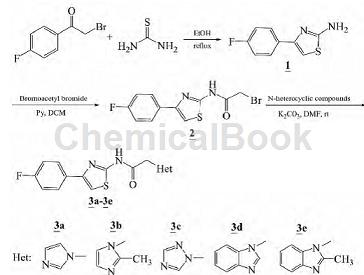
The synthesis of 2-amino-4-(4-fluorophenyl)thiazole (1) is as follows: weigh 2.17 g (10 mmol) 2-bromo-4′-fluoroacetophenone and 0.84 g (11 mmol) ) Thiourea was added to a 50 mL round-bottomed flask, 25 mL of absolute ethanol was added, and the reaction was refluxed for 4 h. After TLC detection of reaction completion, concentrate in vacuo. The residue was dissolved in 50 mL DCM and washed with water (30 mL×2). The organic phase was dried over anhydrous sodium sulfate and concentrated to obtain 1.63 g of light yellow powder 1, with a yield of 84%.
3. Synthesis of new phenyl ether benzofuran derivatives. Furan compounds are widely present in a variety of medicinal plants and have attracted much attention due to their various physiological activities, such as anti-tumor, antibacterial, antifungal, antioxidant, etc. At present, a large number of studies have been conducted on the derivatization of benzofuran compounds at home and abroad, which has enriched people’s understanding of this type of compounds. Phenolic compounds have good bactericidal and antioxidant activities. By connecting phenol and benzofuran, compounds with novel structures and further research value are obtained. Starting from salicylaldehyde and 2-bromo-4′-fluoroacetophenone as raw materials, 2-(4-fluorobenzoyl) benzofuran is generated through substitution and cyclization reactions, and then reacted with phenolic compounds to synthesize 6 new phenyl ether benzofuran derivatives (2a ~ 2f):
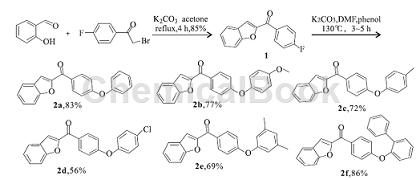
The synthesis of the intermediate 2-(4-fluorobenzoyl) benzofuran (1) is as follows: weigh 2. 44 g (20 mmol) salicylaldehyde and 5. 52 g (40 mmol) K2CO3 in In a 100 mL round bottom flask, add 60 mL acetone, add 4.34 g (20 mmol) 2-bromo-4′-fluoroacetophenone in batches with stirring at room temperature, and heat to reflux for 4 h. After TLC detection, the reaction was completed, cooled to room temperature, and concentrated in vacuo. Add 80 mL of water to the reaction flask, stir for 20 min, and filter with suction. The solid is washed with 20 mL of 10% KOH solution and water (20 mL × 3), and dried to obtain 4.08 g of a yellow-brown solid with a yield of 85%. m. p. 188. 4 ~ 190. 6 ℃;
Main reference materials
[1] Lin Yuping, Hu Chunyan, Zheng Xi, et al. Synthesis of new 4′-(N-substituted-1-piperazinyl) chalcone derivatives and their anti-tumor activity[J]. Chin. J. Org. Chem, 2017, 37: 237-241.
[2] Zhang Mengdi, Mao Zewei. Synthesis of nitrogen heterocyclic substituted aminothiazole derivatives[J]. Fine Chemical Intermediates, 2015, 45(4): 23-25.
[3] Mao Zewei, Jiang Yuan, Rao Kaoxiong. Synthesis of new phenyl ether benzofuran derivatives [J]. Journal of Yunnan University for Nationalities: Natural Science Edition, 2015, 24(4): 270-273.

 微信扫一扫打赏
微信扫一扫打赏

