Background and overview[1][2]
More than thirty years ago, people first obtained sweet dihydrochalcones from citrus flavanones, naringin and neohesperidin. Since then, people have synthesized a large number of derivatives of this sweetener, some of which have only made minor changes in structure, while others have made major changes. All of these are to further understand the relationship between its structure and flavor, or to improve quality, increase solubility and reduce toxicity, etc. Dihydrochalcone is a derivative of flavanones. Its sweetness is about 100 times that of sucrose. It was previously used as an additive for certain drugs, toothpastes, etc. In recent years, research on its physiological functions has proven its health benefits, making its application in beverages and food additives gradually increasing.
Dihydrochalcone (DHC) is a type of compound with a benzochromone core structure. It can be regarded as the product of the C-ring opening of dihydroflavone under alkaline conditions. It has been Isolated from plants such as Liliaceae, Asteraceae, Rosaceae, and Ericaceae. Dihydrochalcones are a very important class of pharmaceutical active ingredients with antioxidant, anti-tumor, anti-diabetic, antibacterial and anti-epileptic effects. Some studies have isolated a dihydrochalcone-loureirin A (loureirin A) from Yunnan blood root, which is 4′-hydroxy-2,4-dimethoxydihydrochalcone, and proved that it has Fungi such as Cladosporium frugivora, Fusarium graminearum var. dracaena, Fusarium graminearum var. Yunnan and Aureobasidium pullulans have inhibitory effects.
Loureirin B is also an important dihydrochalcone. Its chemical structure is 4′-hydroxy-2,4,6-trimethoxydihydrochalcone. It is a traditional Chinese medicine. An important indicator substance in the quality standard of Dragon Blood Dragon’s Blood is also the main active ingredient in liniments such as Hushang’an Tincture, Jianglong Liniment, Yellow Pine Oil, etc. It has the functions of activating blood circulation and removing blood stasis, relieving pain and hemostasis, astringing sores and promoting muscle growth. Studies have shown that teracin B has an analgesic effect on tetrodotoxin-sensitive nanochannel currents in dorsal root ganglion cells by suppressing peak values, inhibiting ion channel conductance, and accelerating inactivation.
Since the content of dihydrochalcone in natural products is very low, and the ingredients in natural products are very complex, separation methods such as traditional organic solvent extraction and column chromatography with ordinary fillers are time-consuming, laborious, and have poor selectivity. , it is difficult to meet the current separation requirements, so studying efficient separation technology has become the focus and difficulty of natural product research and development.
Purpose[3][4]
Dihydrochalcone is a non-nutritive sweetener, suitable for organic intermediates in low-Ph value and low-temperature heating products. Examples of its application are as follows:
1. A method for preparing a compound sweetener with pure taste, which is characterized by combining a steviol glycoside sweetener (ingredient A) and a dihydrochalconic glycoside sweetening enhancer and bitterness inhibitor (ingredient B) Mix it evenly according to a certain formula ratio, or dissolve it in water according to the formula ratio, and then concentrate and crystallize it, or spray-dry it.
The present invention makes full use of the sweetness enhancement and bitterness suppression properties of dihydrochalconic glycosides natural products, overcomes the shortcomings of stevia glycoside sweeteners with post-bitter taste, and makes the compound sweet. The sweetness of the flavoring agent is close to that of sucrose, with good taste, low calories and low cost. It can be widely used in various food and beverage processing.
2. Prepare an edible composition with low glycemic index and pure sucrose taste, including isomaltulose and acesulfame potassium or acesulfame potassium with one or more other high-intensity sweeteners ( A food, edible ingredient or edible composition that is a mixture of HIS). The preparation method is to replace the carbohydrate sweetener with a mixture including isomaltulose and acesulfame potassium or a mixture of acesulfame potassium and one or more other high-intensity sweeteners (HIS).
The new mixture includes a mixture of isomaltulose and acesulfame potassium with one or more other HIS selected from the group consisting of alitame, cyclamate, dihydrochalcone, monk fruit (lohango), neohesperidin, neotame, saccharin, rebaudioside, sucralose and thaumatin.
Preparation[2][5]
Method 1: Almost all dihydrochalcones are derived from the catalytic reduction of chalcones corresponding to triceps, and chalcones are derived from the ring-opening reaction of flavanones under the action of alkali.
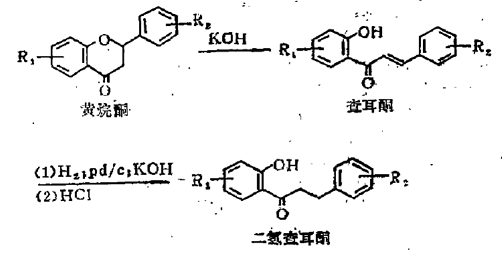
Method 2: From flavonoid glycosides (the content in immature citrus can reach 20%, such as hesperidin), use hesperidinase to cut off the rhamnose residue on the hesperidin sugar group under alkaline conditions. Hydrogenate to obtain glucose hesperetin dihydrochalcone (sweet taste, but solubility is only 0.1%), and then use amyloglucosyltransferase to connect several glucose groups to it, and the solubility reaches 1%. It is commercially available Commercial products include naringin dihydrochalcone and neohesperidin dihydrochalcone. They are obtained by hydrogenation and reduction of benzylidene acetophenone. The reaction is carried out in the solvent ethyl acetate, and the yield is 81-95%.
Main reference materials
[1] Nutritional Science Dictionary
[2] Lu Jingci; Yong Kelan; Huo Shixin; Wang Yuqin; Chen Xu. Preparation method of molecularly imprinted solid phase extraction column packing material CN200810037625.X, application date 20080520
[3] Lin Liwei; Deng Guihong; Deng Cheng; Wu Xiaoliang. A method for making a new compound sweetener. CN201510165184.1, application date 20150409
[4] Susan Ratjen; Susan Schwartz. Edible compositions with low glycemic index and pure sucrose taste. CN200710109538.6, application date 20070625
[5] Liu Shukai, Zheng Jianxian. Dihydrochalone, a non-nutritive sweetener that is struggling [J]. China Beet Sugar Industry, 1991 (5): 39-46.

 微信扫一扫打赏
微信扫一扫打赏

