Background and overview[1][2]
Electrochemiluminescence (ECL) is known for its high sensitivity and low background. As a powerful analytical method, it has been used in the food hygiene and medical industry. The sensitivity of ECL essentially depends on the luminous efficiency of the luminophore. Among them, cathodic electroluminescent substances are dominated by semiconductor nanocrystals, but these substances generally contain toxic components, and the ECL intensity is not high and rely on exogenous strong oxidants as co-reactants for sensitization, which greatly limits the advancement of ECL technology. widely used.
Therefore, it is very necessary to find efficient cathode ECL luminophores and use them to develop simple and stable ECL detection methods. Porphyrin molecules have been widely used in photoelectric sensors and electrochemical analysis due to their special electrochemical activity. The physical and chemical properties of porphyrin nanomaterials are closely related to their morphological and structural characteristics. At present, mixed solvent methods, surfactant-assisted self-assembly (SAS), ion self-assembly, evaporation diffusion methods, etc. have been used for preparation. Porphyrin nanomaterials are synthesized and exhibit a variety of morphological structures at the same time, such as: rhombus, rectangle, spiral, snowflake, etc.
The preparation and assembly of small-sized nanomaterials with special morphologies and simple preparation methods, and regulating their growth processes, have been hot and difficult issues in the study of nanomaterials in recent years. In the final analysis, the most important thing is the controllable growth and assembly of nanomaterials during the preparation process to achieve controllable structure of nanomaterials, thereby achieving the ultimate goal of applying low-dimensional nanomaterials to the device level. Zinc tetraphenylporphyrin can achieve controllable structural morphology, and its unique structural system and physical properties play a prominent role in the fields of nanoscience and biological science.
Structure
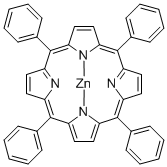
Apply[2][3]
Metal porphyrin complexes (Porphyrin) such as zinc tetraphenylporphyrin are planar macrocyclic molecules with a highly conjugated π-electron system. They have good light and thermal stability. They operate in the visible light region (400~ 700nm) has strong absorption, giving it excellent properties in catalysts (including photocatalysts), photovoltaic cells, dyes, optical recording materials, biomedical materials, etc. Therefore, metalloporphyrin complexes have been hailed as new materials in the 21st century, and related basic and applied research has become a recent research hotspot.
Metalloporphyrin complexes with hydrophilic or hydrophobic substituents are used as photoactive substances and attached to the surface of ZnO nanofilms and ZnO nanoparticles respectively to broaden the spectral response and improve lighting efficiency, photoelectric conversion efficiency, and light Catalytic activity and photocatalytic selectivity. Application examples:
1. Preparation of a zinc tetraphenylporphyrin/zinc oxide composite film nanomaterial
, the assembly steps are: 1) Synthesize bird’s nest-shaped ZnO nanofilm material on ITO conductive glass; 2) Synthesize tetraphenylporphyrin (H2TPP); 3) Dissolve tetraphenylporphyrin in chloroform solvent 4) Dip the ZnO nanofilm material attached to the ITO conductive glass into the H2TPP solution to achieve uniform spin coating and adhesion immediately; 5) Place it in a tube furnace and calcine in nitrogen.
During the preparation process, zinc tetraphenylporphyrin (ZnTPP) is self-assembled in situ on the surface of ZnO. The obtained organic and inorganic composite materials have clean interfaces, chemical bonding, and good stability, which not only broadens the scope of composite materials The visible light absorption spectrum can also improve the separation efficiency of photogenerated charges, greatly improve the photocatalytic degradation efficiency, and show hydrophobicity and obvious selectivity for organic dyes.
2. Prepare a halogen-free low-smoke flame retardant for preparing polyethylene materials.
Mainly composed of dimethyl methyl phosphonate, sodium stearate modified Mg(OH)2 (commercially available), 2,3-dimethyl-2,3-diphenylbutane, slope It is compounded of hegorite clay and zinc tetraphenylporphyrin. The polyethylene material prepared by using this compound flame retardant has an oxygen index of no less than 32, a smoke density of no more than 75 under flameless conditions, a tensile strength of no less than 12MPa, and an elongation at break of no less than 180%. A modified halogen-free low-smoke flame-retardant polyethylene composite material with very good performance. This material can be widely used in wires and cables, construction and other fields.
If the prepared product requires cross-linking treatment, the flame retardant properties of the material will not be affected after conventional irradiation cross-linking, and its mechanical properties will be further improved.
Preparation[2]
Step 1: Add 60mL propionic acid, 20mL nitrobenzene, and 3.85mL benzaldehyde to a three-necked flask equipped with a reflux device and a constant pressure dropping funnel. Stir and heat. When the droplets begin to reflux, the temperature is about 140°C. Add 2.1 mL of freshly steamed pyrrole and 15 mL of nitrobenzene mixture dropwise through the constant-pressure dropping funnel while stirring, and continue to react under reflux for 4 hours. After the reaction stops, cool and let stand in the refrigerator overnight, and filter with suction using a Buchner funnel. Dry it in an oven to obtain the crude product tetraphenylporphyrin;
Step 2: Fully dissolve the crude product (4g) obtained in step (:1) in chloroform (500mL), add about 1g of DDQ in 35mL of dry benzene, stir the mixture and reflux for 3h, and use the light yellow solution with a Filter activated alumina through glass sand (60g Al2O3 and CH2Cl2 are mixed, and then Place it flat in a glass gauze funnel and cover it with a piece of filter paper), wash the filter cake with CH2Cl2, combine the washing liquid and filtrate, concentrate to 30mL, add 6mL methanol, Cool to room temperature and filter to obtain ���Purity tetraphenylporphyrin (H2TPP);
Step 3: Take 0.05g of tetraphenylporphyrin into a beaker, and add chloroform drop by drop while stirring until the tetraphenylporphyrin is completely dissolved to form a tetraphenylporphyrin solution
Main reference materials
[1] Wang Hai; Yang Yiji; Zhang Jun; Zhao Jianhong; Wang Xiaoyan; Yu Leiming; Liu Ying. A method for preparing zinc tetraphenylporphyrin nanomaterials with strong spectral absorption capacity and high carrier mobility. CN201610030408.2, application date 20160118
[2] Pan Haibo; Huang Huihan; Zhang Junxian; Shen Shuifa; Jiang Rongx. In-situ self-assembly preparation method of zinc tetraphenylporphyrin/zinc oxide composite film nanomaterials. CN201610030408.2, application date 20161111
[3] Lei Ziqiang; Ma Delong; Zhang Zhe; Zhao Rui; Yang Zhiwang. A halogen-free low-smoke flame retardant for the preparation of polyethylene materials. CN201510948649.0, application date 20151217

 微信扫一扫打赏
微信扫一扫打赏

