Background and overview【1】
Trimethylhydroquinone is an important intermediate for the industrial synthesis of vitamin E. Vitamin E is obtained by condensation reaction with isophytol side chain. As an important antioxidant, vitamin E is mainly used as an additive in medicine, food, feed and cosmetics. Currently, the vitamin E products of the four major German companies BASF, Dismann, Zhejiang NHU and Zhejiang Pharmaceutical occupy 95% of the global market share.
Application【1】
With trimethylhydroquinone as the main ring and the side chain C isophytol, the vitamin can be obtained by heating and condensing it in ethyl acetate with sulfuric acid as the condensing agent. In recent years, due to the wide application of vitamin E in the fields of medical treatment, food and feed, vitamin E products have continued to be popular around the world, especially in the European and American markets, where demand exceeds supply and prices are firm. Demand for trimethylhydroquinone, used in the production of vitamin E, will also follow.
1. Pharmaceutical industry
Vitamin E is the general name for tocopherol compounds, also known as tocopherols, reproductive vitamins, anti-infertility vitamins, etc. It appears as a golden or light yellow viscous oily liquid with a mild, special smell and taste, and a relative density of 0.947-0.955. It is easily oxidized and turns dark red when exposed to air and light. It is miscible with acetone, ether or plant koji, is easily soluble in ethanol, and is almost insoluble in water. It is a fat-soluble antioxidant. Products can be divided into two categories: natural and synthetic. The most commonly used form of vitamin E is its acetate, and its preparations can be divided into oils and powders.
In recent years, people have made rapid progress in research on the medical functions and effects of VE. As an intracellular antioxidant, VE can inhibit the redox reactions in various cells and organs, and can especially protect cell membranes from Attack by free radicals generated by peroxidation of unsaturated vinegar compounds. Studies have shown that the lack of VE in the human body will directly affect the normal functions of reproductive, muscle, circulatory, skeletal and nervous systems. VE can also reduce the damage of various toxins to the human body. In the pharmaceutical industry, vitamin E is used as a drug to reduce the occurrence of heart disease, reduce chronic intestinal inflammation, and resist aging. It can also prevent human lung bleeding, liver necrosis, kidney disease, creatine urea and other diseases, and enhance immunity. The demand for VE in medicine has grown rapidly in recent years.
2. Food
VE added to food can play the role of antisepsis and preservation. As a food antioxidant, VE has many advantages, such as high boiling point and good thermal stability. Therefore, it is especially suitable for foods that need to be heated and preserved, such as instant noodles, margarine, and milk powder. wait. VE is more suitable for the production of various health-fortified foods, especially as a nutritional fortifier for infants and pregnant women.
In the food industry, vitamin E is mainly used as a good antioxidant in fatty and oily foods, which can maintain the fresh flavor of processed foods and make them stable and lasting. Adding 0.04% vitamin E in fish processing can improve fish odor and product flavor. Adding 0.05% vitamin E to the raw meat of processed sausages can keep the sausages fresh and preservative. Vitamin E is also used in the production of various functionally fortified foods, especially as a nutritional fortifier in infant and young child food.
Recently, it has been discovered abroad that an important industrial use of vitamin E is to replace traditional antioxidants such as BHA and BHT as an antioxidant additive for plastic products. At present, most food packaging bags are made of plastic films. The industrial antioxidants added in them are toxic to the human body to varying degrees. The use of vitamin E as a non-toxic substitute for plastic antioxidants has been proven to be a safe and effective stable system for food. It has no impact on the color, aroma and taste, and can provide antioxidant protection to keep food from spoiling for a long time.
3. Cosmetics
Environmental pollution and ultraviolet radiation will produce free radicals, causing damage to skin, cells and tissues, and accelerating the aging process. Studies have confirmed that VE plays a decisive role in protecting the skin from free radical damage. At the same time, VE acts as an antioxidant and can extend the use time of cosmetics. VE can promote skin metabolism, prevent pigmentation, improve skin elasticity, and has beauty, skin care, anti-aging and other properties. It has become the mainstream of nutritional cosmetics in the international market.
Vitamin E is a substance needed by human skin cells. It also has antioxidant and anti-aging effects. However, normal vitamin E is difficult to be absorbed by cells through the skin; but when vitamin E is treated with nanotechnology, it can be absorbed within a few minutes. It can penetrate the dermis and be quickly absorbed by skin cells, and has the function of removing freckles. This nano-vitamin E cosmetic is more effective and faster than ordinary freckle-removing products containing hydrogen compounds, and it is safe and has no toxic side effects. In this way, nano-cosmetics have taken a big step forward in solving the problem of stubborn freckles. It uses a brand-new method to solve the problem that general anti-freckle ingredients are difficult to be absorbed by the skin.
Preparation of methylhydroquinone【2】
1 Mesitylenol method
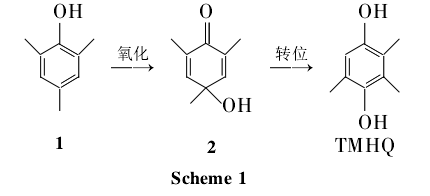
The mesitylenol method uses NaOH as a catalyst, and mesitylene (1) is oxidized into 4-hydroxy-2,4,6-trimethyl-2,5-cyclohexadienone (2) in high-pressure oxygen. ; 2 was synthesized by methyl translocation at 250°C and then reduction to synthesize TMHQ (Scheme 1), with a total yield of 47%. l can be synthesized by methylation of phenol or extracted from the by-product of the synthesis of 2,6-dimethylphenol, so the mesitylenol method has great practical value.
This method has a short process flow, but the price is relatively high, and it is difficult to achieve large-scale production by relying only on the extraction of 2,6-methylphenol by-products. At present, the United States and Japan mostly adopt�The production route.
2. Phenol method
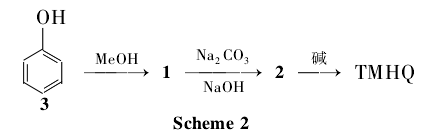
After phenol (3) and methanol [n(phenol):n(methanol)=1:5] are vaporized and mixed respectively, they enter the two-stage fixed-bed isothermal reactor and conduct gas-solid phase at 450℃-500℃. Catalytic reaction, 1 is obtained through condensation and distillation; 1 is passed into sodium carbonate and sodium hydroxide solutions with steam to undergo Bamherger transposition reaction to obtain 2; finally treated with alkali to obtain TMHQ (Scheme 2), with a total yield of 60%.
This method is actually the recycling of by-product 1 of the process of producing 2,6-dimethylphenol (monomer for synthesizing PPO heat-resistant resin). Currently, the United States, Japan and other countries use this process to produce TMHQ.
3. Isophorone oxidation method
In the isophorone oxidation method, acetone (13) is first polymerized into isophorone (14); 14 is oxidized through rearrangement to obtain oxyisophorone (16); 16 is obtained through phthalization and rearrangement reactions. Trimethylhydrodiacetate (17); 17 is saponified and hydrolyzed to obtain TMHQ (Scheme 8).
The raw materials of this method are cheap and easy to obtain, the process is simple, the environmental pollution is small, and it is convenient for large-scale production. It is a green and environmentally friendly production process. However, the reaction route is long, the operation is cumbersome, and the overall cost is high. In addition, researchers from Germany, the Netherlands and other countries have applied for a large number of patents in China, which will also increase the domestic production cost of vitamin E.
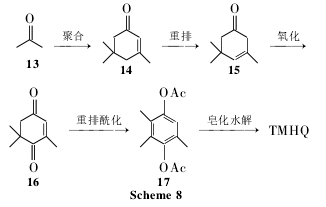
The key technology of this process lies in the oxidation of 14 and the rearrangement and phthalation of 16. At present, the oxidation of Yuepingphorone mainly uses organic coordination compounds of transition metals and metal-free catalytic systems as catalysts, and is oxidized with molecular oxygen or air, and an appropriate amount of additives (such as co-solvents, etc.) are added. The catalysts used mainly include: transition metal salt catalysts, transition metal Schiff base catalysts, transition metal acetyl acetone complex catalysts, ionic liquid supported acetyl acetone metal complex catalysts, transition metal phyllans or phthalate accumulators. Complex catalysts, all-metal catalysts and metal-free catalytic system catalysts, etc. Martin Jochen Klatt et al. used Schiff base and potassium acetate or hinge acetate as catalysis. This method easily produces 3,5,5-trimethyl-cyclohexan-2-en-1-one and 2,2,6-trimethyl By-products such as cyclohexane-1,4-dione greatly reduce the selectivity of the reaction and the yield of oxyisophorone. Moreover, the properties of these by-products are similar to the products, and it is quite difficult to separate them from the products; Nohuhiko Ito et al. used iron , cobalt, copper, manganese leaf or phthalate complexes as catalysts. This method has a high yield, but the catalyst is quite expensive and is easily toxic and deactivated during the reaction, resulting in high cost of the process.
Compared with β-isophorone, there is an enol conjugate system in the a-isophorone structure, which has high stability and low reactivity. It is difficult to synthesize 16 by direct catalytic oxidation. So far, the catalytic oxidation of a-isophorone can be divided into two categories according to different catalytic systems: homogeneous catalytic systems and heterogeneous catalytic systems. Homogeneous catalytic systems include: phosphoplatinic acid or silicoplatinic acid CuSO4, catalytic system, phosphoplatinic acid/dimethylsulfoxide/potassium tert-butoxide catalytic system, metal cold hydroxybenzaldehyde complex Compounds, vanadium acetate acetonate, sodium vanadate, tetraphenylpyralemanganese chloride and N-hydroxyphthalimine//CuCl2, etc. Heterogeneous catalytic systems include: supported metal ligands, nail-loaded magnesium-aluminum hydrotalcite, Cu/Co/Fe-loaded magnesium-aluminum hydrotalcite and platinum-vanadium phosphate-loaded activated carbon, etc. In the presence of a catalyst, 16 reacts with a phthalating agent (such as phthalate, phthalate halide or enol ester) to form TMHQA, and then undergoes saponification reaction to obtain trimethylhydrogenaldehyde acetate (TMHQ 1 VIA) or TMHQ.TMHQ -VIA can directly react with isophytol to form acetamine E, the main component of vitamin E. Traditional catalysts for rearrangement and phthalation are Lewis acids and Brucella acids, such as HF, trifluoromethanesulfonic acid, chlorosulfonic acid, polyphosphoric acid, fuming sulfuric acid and mixtures of these acids. This type of catalyst has high reactivity, but is too corrosive, easily forms acid gas flow, and generates a large amount of salt after the neutralization reaction, which is not conducive to product purification and purification. Solid acid has received widespread attention because it is not easy to corrode equipment and can be easily separated and recycled after the reaction. The more studied solid acid catalyst is indium salt, preferably trivalent indium salt, such as InC13 and perfluorinated sulfonic acid resin. This type of catalyst has the same high activity as sulfuric acid and can achieve 100% conversion of raw materials, but it is not resistant to high temperatures, has weak stability, and is not easy to reuse. The current research focus is on high-fluorine ion exchange resins with excellent catalytic properties, such as Nafion NR 50 (polymerized by tetrafluoroethylene and CFZCF-S03 H), whose acid content is 0. 95 mmol·g-1 , the chemical surface area is only 0. 02 m2·g-1, and the catalytic performance is stable, but the reaction activity in the gas phase and non-swelling solvent is low. At present, there is a catalyst that combines Nafion and soluble Si02:, which has a temperature resistance of up to 320°C, reduces environmental pollution, facilitates large-scale industrial production, and has good application prospects. .
Main references
[1]Wang Wen. Development and application of trimethylhydroquinone[J]. Fine Chemical Raw Materials and Intermediates, 2004(12):16-18.
[2] Bai Yuansheng, Li Zicheng, Wang Lixin, Peng Lin, Xu Xiaoying. Research progress on the synthesis of 2,3,5-trimethylhydroquinone [J]. Synthetic Chemistry, 2014, 22(03): 423-428 .

 微信扫一扫打赏
微信扫一扫打赏

