Background and overview[1][2]
Monobenzone, chemically named 4-(Benzyloxy)phenol, is a depigmenting agent that can enhance the elimination rate of melanin secreted by skin cells. Effect, research shows that the molecular mechanism of its effect may be related to tyrosinase. Moreover, clinical experiments have also proven that monobenzone can effectively treat vitiligo, stains, pigmentation and other diseases. Monobenzone is a topical depigmenting agent used to treat hyperpigmentation that blocks the production of melanin in the skin without destroying melanocytes.
Preparation[3]
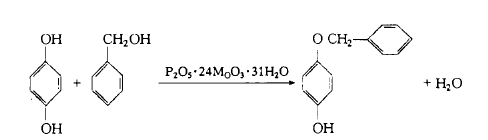
Weigh 4.8g of hydrogen alkali, add 20g of benzyl alcohol, stir and dissolve, add 1g of phosphomolybdenum acid catalyst at 120°C, during the reaction process, use air with a flow rate of 45 ml/min to remove the catalyst and the reaction process crystallization water produced. Stir continuously. After the reaction is completed, 20g toluene and 10g water are added to the reaction solution. At about 80°C, the product hydrogen monokyl ether is transferred to the toluene solvent layer, and unreacted benzyl alcohol remains in the water layer (separated and removed). The organic layer was evaporated to remove toluene, then 60 ml of water was added, cooled, filtered, and dried to obtain the crude product, which was then recrystallized with alcohol and water to obtain 8.4g of milky white crystals, with a yield of 9 2%
Apply[4]
Benoquin Cream 20% is indicated for vitiligo with extensive eventual depigmentation. Benoquin Cream 20% is intended for permanent topical application to discolor vitiligo lesions that have spread around normal skin (more than 50% of the body surface area) in patients with idiopathic vitiligo.
Monobenzone is a topical depigmenting agent used to treat hyperpigmentation, such as various stains, age spots, melanoma, etc. The effect is very obvious. It can decompose melanin in the skin, prevent the production of melanin in the skin, and restore the healthy color of the skin without destroying melanocytes. It is very toxic and is usually made into ointments or liniments. It has been included in the United States Pharmacopeia. At present, there are no drugs for the treatment of spots in our country, and the cosmetics used for the treatment of spots have little effect, and some cosmetics add large amounts of harmful substances such as hydroquinone and metallic mercury to achieve the effect of removing spots and whitening. Developing monobenzone into anti-freckle medicines or cosmetics has good effects and low toxicity, which can meet the urgent needs of a large number of yellow people suffering from spots, and will be welcomed by domestic and foreign markets. The ex-factory price of each ointment (ointment or cosmetic ointment) is 80 yuan, and the cost of each tube is 12 yuan. The annual production scale is 3 million pieces, the output value reaches 240 million yuan, and the annual net profit is 120 million yuan. The total investment is about 11 million yuan, and profits can be made that year. The results of this product have been tested on the pigmented spots and age spots on the faces of trial users, showing that it is a highly efficient and low-toxic product. In addition to being developed into medicines, monobenzone can also be developed into cosmetics. It is simple to apply for approval and has quick results, so its application prospects are very good. However, as a new drug development, companies still need to invest considerable financial support to complete pharmacological and clinical trials and obtain the national new drug certificate.
Quality Control[5]
1. Properties: This product is a milky white or slightly yellow, uniform and delicate O/W type cream. Meets the ointment requirements of the 2005 edition of the Chinese Pharmacopoeia.
2. Identification: Take an appropriate amount of this product, equivalent to 500 mg monobenzone, into a centrifuge bottle, add 100 ml of water, shake until the cream is completely dispersed, centrifuge, and gently pour out the material floating on the surface. Wash the filter residue with water, centrifuge again, and gently pour out the water layer. Transfer the residue to a separatory funnel, add chloroform to adjust the volume to about 100 ml, shake, separate the chloroform layer, and filter with cotton gauze. Transfer the filtrate to a 150 ml flask, evaporate in a ventilated place to recover chloroform, add 5 ml pyrimidine and 3 ml anhydrous acetic acid to the dry residue, reflux the flask for 10 min, cool, then add 100 ml water and 6 ml acetone , seal the flask and cool it in the refrigerator for 1 hour. Separate the precipitate in a glass crucible. Wash the precipitate with water until there is no residual pyrimidine smell. Dry the precipitate with phosphorus pentoxide in a vacuum desiccator for 16 hours. The melting point of the precipitate should be determined to be 110 to 113°C (monobenzone acetate).
3. Inspection: It should comply with the relevant regulations under the cream category of the 2005 edition of the “Chinese Pharmacopoeia”.
4. Content determination: chromatographic conditions EclipseXDB – C18 (150 mm × 4.6 mm, 5 μm) chromatographic column, column temperature 30℃, detection wavelength 292 nm, mobile phase: acetonitrile: water =60:40, flow rate 1.0ml/min , injection volume 10 μl
5. Sample stability and irritation test
1) Light test: Place this product in a colorless glass vessel, expose it to (4500 ±500) Lx strong light, and take samples on 0, 1, and 5 days to examine the properties, melting time, content and other indicators. As a result, compared with 0 d, all indicators of the sample were slightly darker in color and slightly lower in content, indicating that this product is sensitive to light and should be stored away from light.
2) Accelerated test: Take 3 batches of cream and place them under the conditions of (30 ±2)℃ and relative humidity (60 ±5)% for 10 days, and place them at 0, 1, and 5 respectively. The properties, content, etc. were examined on 10 days and 10 days, and there was no significant change in all indicators compared with 0 days.
Irritation test: Take 10 healthy adult mice, regardless of gender, weighing (20 ±3) g, and divide them into a drug administration group and a blank control group (blank matrix). The hair on the back is shaved off, and the ointment is applied on On the back of the mouse, the local skin reaction was observed 24 hours later. As a result, there was no redness or swelling on the back, and there was no significant difference between the two groups.
Since the cream contains 20% monobenzone, and since monobenzone is a fat-soluble drug, the dosage of other components in the oil phase should be appropriately reduced. The saponification product of stearic acid and triethanolamine is an O/W emulsifier. Stearyl alcohol is used as an auxiliary emulsifier and lubricant, which can emulsify and absorb more water. Glycerin is used as a moisturizer. Azadrone is a colorless and odorless emulsifier. Liquid, it feels slippery when in contact with the skin, non-irritating and non-toxic. It can make the cream soft and easy to spread. It can also significantly enhance the speed of the drug passing through the stratum corneum of the skin and increase the concentration of the drug in the skin, thereby better performing its treatment. It is a good penetration enhancer. It uses an oil-in-water cream base, has simple preparation process, reasonable design, good stability, non-irritation, and good clinical efficacy, and is worthy of clinical promotion and application.
Main reference materials
[1] CN201510675321.6 Use of monobenzone in the preparation of medicines
[2] Zhang Aihua, Zhang Yuehan, Chen Xinju. Determination of monobenzone content by RP-HPLC method [J]. Journal of Pharmaceutical Practice, 2003, 21(6): 350-351.
[3] Sun Zhongfan, Zhao Quanqin, Wang Defeng, et al. Improvement of the synthesis of monobenzone [D]. , 1997.
[4] Powerful freckle remover—monobenzone
[5] Yu Botao, Kang Li. Preparation and quality control of monobenzone cream[J]. Southwest National Defense Medicine, 2009, 19(11): 1114-1116.

 微信扫一扫打赏
微信扫一扫打赏

