Background and overview[1][2]
Dilute solutions of acridine and its salts fluoresce purple or green. The dilute solution of salts has green fluorescence, and when diluted again, due to the hydrolysis of the salt, it becomes free acridine, which exhibits purple fluorescence. The aqueous solution is weakly alkaline and reacts with inorganic acids to form salts. It reacts with halogenated hydrocarbons or halogenated aromatic hydrocarbons to form quaternary ammonium compounds that produce color. Acridine is very stable, has a similar structure to anthracene, and has very similar chemical properties. Both the vapor and the solution are irritating and can strongly irritate the skin and mucous membranes. Inhaling the vapor can cause coughing. Acridine is an important class of organic nitrogen-containing heterocyclic compounds, which was first extracted from coal tar by Gabriel in 1874. The tricyclic rigid planar structure gives acridine strong aromaticity, and due to the large angular tension of acridine, it has good reactivity. Because the aromatic structure of acridine can be embedded into the double helix structure of DNA, it has good biological activity. Acridine derivatives have been widely studied and applied in anti-tumor, anti-viral, anti-malarial and bioluminescent probes. .
Apply[2][3][4]
Acridinium salts are widely used and can be used as photocatalysts, preparation of dyes, etc. Examples of its application are as follows:
1. Used as photocatalyst. Preparation of Ponatinib, an anticancer drug. In December 2012, it was approved by the US FDA for the treatment of adult chronic myelogenous leukemia (CML) and “Philadelphia chromosome positive” (Ph+) acute lymphoblastic leukemia (ALL).
There is research to provide a preparation method for ponatinib to solve the technical problems of existing ponatinib synthesis methods using expensive catalysts, high costs, and unsatisfactory yields of target products. The preparation method starts from 3?halo?pyrazolo[1,5-a]pyrimidine, sequentially uses trimethylsilyl acetylene, tetrabutylammonium fluoride, etc. as raw materials, and uses acridine salt as a photocatalyst in copper Salts and alkaline organic solvents are used to construct a reaction system, thereby making the synthesis route of ponatinib indirect, with mild synthesis conditions, low cost, and high yield of ponatinib. The acridinium salt photocatalyst has high catalytic efficiency in the reaction system provided by the alkaline organic solvent containing copper salt, thereby avoiding the existing need to use expensive palladium catalysts, thereby effectively reducing the cost of the catalyst used in the synthesis. The molar ratio concentration of the compound A and the acridinium salt photocatalyst is controlled to 100: (1-5). Since the acridine salt, such as the acridine salt of the above structural formula, is used as the catalyst, the usage amount of the acridine salt is effectively reduced and the photocatalytic efficiency is improved.
There is also research on using acridine salt as a catalyst to prepare zaleplon. Zaleplon (Zaleplon), the chemical name is 3-[3-cyanopyrazole (1,5-α) pyrimidine-7] -N-Ethyl acetanilide is a non-benzodiazepine sedative-hypnotic drug produced and launched by Wyeth Company of the United States in March 1999. Zaleplon is a complete agonist of benzodiazepine (BZ) ω1-type receptors. It acts on the benzodiazepine GABA subtype A receptor complex and produces central inhibitory effects. It is highly selective for ω1-type receptors. It can also bind to omega-type receptors, but not other neurotransmitters. In August 1999, the US FDA approved zaleplon for the treatment of adult insomnia.
2. Prepare dye. Studies have provided a type of acridinium salt dye that uses double bonds to connect 9,10-dimethylacridinium salt with different thiophene or benzene groups and serves as a channel for charge transfer between the two. , forming a SERS dye compound with a D-π-A structure. The general structural formulas of the acridinium salt dye are shown in (1) and (2):
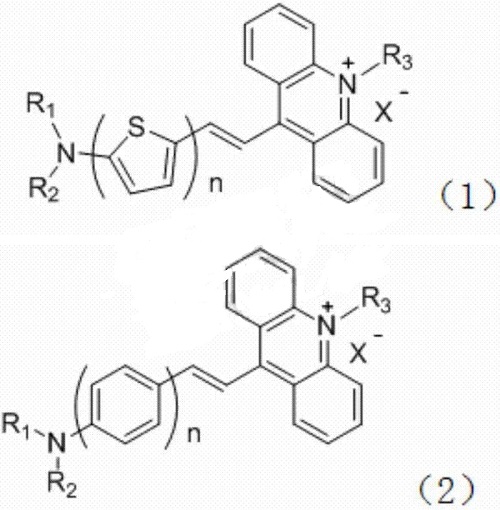
The preparation method of the acridinium salt dye is as follows:
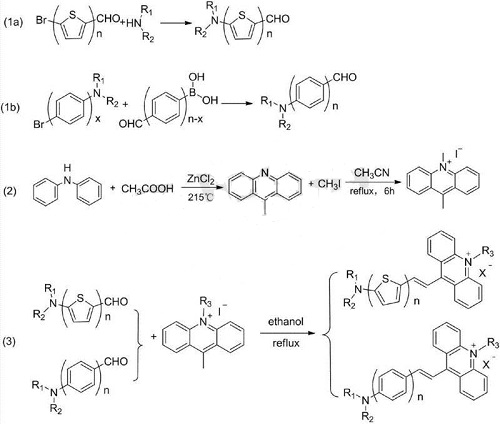
Specific steps include the following:
(1a) The reflux reaction of n-bithiophene with bromine and formaldehyde as substituents at the terminal 5-position and a dialkylamine aqueous solution produces n-bithiophene with the terminal 5-position dialkylamino group and formaldehyde as substituents;
(1b) The reflux reaction of x-biphenyl with dialkylamine and bromine as substituents at the terminal 4-position and n-x biphenyl with formaldehyde and boric acid as substituents at the terminal 4-position respectively produces a dialkyl group at the terminal 4-position. n-biphenyl with amine group and formaldehyde as substituents;
(2) Diphenylamine and acetic acid react under the action of zinc chloride to form 9-methylacridine, which then reacts with an alkyl halide in acetonitrile to form 9,10-dimethylacridine. Iridinium salt;;
(3) n-bithiophene with a dialkylamino group and formaldehyde as substituents at the terminal 5 positions respectively or n-biphenyl with a dialkylamino group and formaldehyde as a substituent at the terminal 4 positions and 9,10- The dimethyl acridine salt reacts to generate the n-bithiophene or n-biphenyl acridine salt.
Main reference materials
[1] Practical Fine Chemical Dictionary
[2] CN201710272172.8 A type of acridinium salt dye and its preparation method and application
[3] CN201711489758.6 Preparation method of ponatinib
[4] CN201711498737.0 Preparation method of zaleplon

 微信扫一扫打赏
微信扫一扫打赏

