Background and overview[1][2]
Photoinitiator refers to a substance in a photopolymerization imaging system that is directly sensitive to light and promotes the polymerization of monomers into polymer compounds. It can improve the sensitivity of photopolymerizing materials, expand the color range, and play a spectral sensitization role. Commonly used compounds include the following: carbonyl compounds (benzophenone, polynuclear quinone), with a color sensitive range of 360 to 420 nanometers; azo compounds (azobisisobutyronitrile, diazonium salts), with a color sensitive range of 340 nm ~400 nanometers; organic sulfur compounds (thiols) have a color sensitivity range of 280 to 400 nanometers; halides have a color sensitivity range of 300 to 400 nanometers; sensitizing dyes have a color sensitivity range of 400 to 700 nanometers; zinc oxide has a color sensitivity range Wavelength 300~380 nanometers.
In light-initiated curing or polymerization systems, such as UV-curing inks, varnishes, adhesives, and silicones, this chemical can break certain chemical bonds when exposed to light. Reactive groups are formed, which then initiate polymerization or cross-linking reactions. Generally called a catalyst. Some UV-curable silicone systems are catalyzed with photosensitive chelates. UV radiation breaks the chelates, causing them to initiate a curing reaction.
1-Hydroxycyclohexyl phenyl ketone, also known as photoinitiator 184, is an α-hydroxyketone photoinitiator, the chemical name is 1-hydroxycyclohexyl phenyl ketone, α-hydroxyketone photoinitiator It is a new type of high-efficiency photoinitiator. Since there is no α-hydrogen atom, this type of photoinitiator system has high stability, long storage life, wide UV absorption range, fast polymerization speed, good solubility, low odor, and low pollution. The prepared photosensitive composition has excellent stability and is widely used in coatings, inks, electronics, optical fiber, adhesives, printing and other fields, and shows strong vitality.
Apply[3][4][5]
Photoinitiator 184 is widely used in coatings, inks, electronics, optical fibers, adhesives, printing and other fields, and shows strong vitality. Examples of its application are as follows:
1. Prepare a UV-curable coating for battery car outer shell.
The ultraviolet curable coating is composed of a primer and a topcoat. The components of the primer and the topcoat are respectively by weight: primer: epoxy acrylate 20~40, polyurethane acrylate 10~ 20. 1,6-hexanediol diacrylate 2~6, trimethylolpropane triacrylate 30~40, 1-hydroxycyclohexylphenylmethanone 1~5, benzildimethyl ketal 3 ~8, polyether modified polydimethylsiloxane 0.2~1; topcoat: acrylate modified solvent-containing oligomer 40~60, trimethylolpropane triacrylate 30~50, 1-hydroxy Cyclohexyl phenyl ketone 5~10, 2,4,6-trimethylbenzoyl-diphenylphosphine oxide 1~3, polyether modified polydimethylsiloxane 0.5~2, trifunctional acid Esters 1 to 3, nanoscale color essences 1 to 3. The preparation method is:
1) Preparation of primer: Add epoxy acrylate, polyurethane acrylate, 1,6-hexanediol diacrylate, trimethylolpropane triacrylate, and 1-hydroxyacrylate according to the above primer composition ratio. Cyclohexyl phenyl ketone, benzyl dimethyl ketal, and polyether modified polydimethylsiloxane are dispersed and stirred at high speed. The temperature is controlled at 20 to 25°C and stirred at 800 to 1000 rpm for 0.5 After h~1h, mix evenly, filter and remove slag, and the finished primer is ready;
2) Preparation of topcoat: Add acrylate modified solvent-containing oligomer, trimethylolpropane triacrylate, 1-hydroxycyclohexyl phenyl ketone, 2, 4,6-trimethylbenzoyl-diphenylphosphine oxide, polyether-modified polydimethylsiloxane, trifunctional acid ester, and nanoscale color fines are dispersed and stirred at high speed, and the temperature is controlled at 20 to 25°C. , stir at 800~1000 rpm for 0.5h~1h, mix evenly, filter and remove slag, and the finished topcoat is ready.
2. Prepare a UV resin for producing reflective films.
By weight, including 25%-50% bisphenol A epoxy acrylate, 25%-50% trimethylolpropane triacrylate, 18%-33% tetrahydrofuran acrylate, 1%-5%1 -Hydroxycyclohexyl phenyl ketone and each component are prepared according to the above weight ratio. In an environment with good ventilation and no strong light exposure, first combine trimethylolpropane triacrylate, bisphenol A epoxy acrylate, and tetrahydrofuran acrylate. The ester and 1-hydroxycyclohexyl phenyl ketone are added to the mechanically stirred reactor in sequence, stirring while adding, stirring for 2 hours to 3 hours, and finally the discharging valve controls the slow discharging, metering, and packaging. The present invention It is easy to prepare, has low cost, high mold release, high leveling, high hardness and yellowing resistance, and can meet the requirements for producing high-performance reflective films using UV coating methods.
3. Preparation of matte anti-static coating material.
The matte anti-static coating material is used for release paper. The matte anti-static coating material consists of the following mass percentage components: 2% silica particles and an average molecular weight of 500~2000 28% acrylic polyol, 36% resin of polyoxyalkylene series polyol with an average molecular weight of 200 to 2500, 20% polyethylene glycol, 4% cyclomethicone, 1-hydroxycyclohexylphenylmethanone- The photoinitiator 184 is 10%; the particle size of the silica particles is that the particle size distribution measured by the laser diffraction and scattering method has a peak value in the range of 0.1~2 μm and 1.5~5 μm respectively, and the average particle size is 4 μm or the following. The invention not only solves the problem of low bonding force and unstable peeling force between the coating layer of the release paper and the release agent coating, improves the scratch resistance, but also improves the antistatic performance of the film surface.
Preparation[6][7]
Method 1: The specific method of photoinitiator 184, including the following steps:
1) Cyclohexylcarboxylic acid chloride undergoes an electrophilic substitution reaction with benzene under the catalysis of Lewis acid to obtain cyclohexyl phenyl ketone;
2) Cyclohexyl phenyl ketone reacts with hydrogen peroxide and hydrochloric acid solution under the action of a catalyst to obtain 1-chlorocyclohexyl phenyl ketone;
3) The chlorinated intermediate undergoes hydrolysis reaction with sodium hydroxide aqueous solution under the action of a catalyst to obtain 1-hydroxycyclohexyl phenyl ketone.
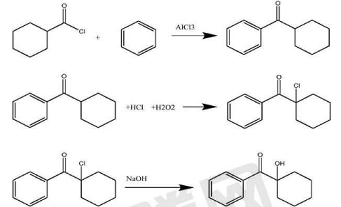
Using the hydrogen chloride in the hydrochloric acid by-product during the synthesis of alkyl ketones, using an L potassium molecular sieve catalyst to perform chlorination and hydrolysis in hydrogen peroxide and organic solvent systems to form α-hydroxyketones. This new synthesis method has The following advantages: The raw materials are cheap and easy to obtain, safe and environmentally friendly. It makes full use of the hydrogen chloride generated when the acid chloride performs the electrophilic substitution reaction under the catalysis of Lewis acid, and recycles it, reducing the discharge of waste acid water and being environmentally friendly. Selective chlorination and hydrolysis are completed in the same kettle, with simple operation, low loss and high yield.
Method 2: A synthesis process of 1-hydroxycyclohexyl phenyl ketone, which is characterized by the following steps:
1) Acylation: Add cyclohexylcarboxylic acid into the reaction kettle, raise the temperature to 35±2℃, add phosphorus trichloride, incubate at 60±2℃ for at least 4 hours, then separate the inorganic layer to obtain cyclohexanemethane Acid chloride; wherein, the weight ratio of the raw material cyclohexylcarboxylic acid and phosphorus trichloride is 1.8-2.2:1;
2) Friedel-Crafts reaction: Add benzene and catalyst aluminum trichloride into the reaction kettle, cool to 5-10°C, then add dropwise the cyclohexanecarboxylic acid chloride prepared in step (1), benzene, trichloride The weight ratio of aluminum to cyclohexanecarboxylic acid chloride is 9:2.9-3.1:3.16-3.36, at 15±2°C for at least 2.5 hours, to obtain a complex of cyclohexyl phenyl ketone and aluminum trichloride;
3) Hydrolysis reaction: Add an appropriate amount of dilute hydrochloric acid with a mass concentration of 1.5-2.5% into the complex, stir and separate the layers, separate the aqueous layer containing aluminum trichloride, and then add an appropriate amount of water to wash the acid to the pH > End at 5 o’clock, and finally remove the unreacted raw material benzene through distillation to obtain cyclohexyl phenyl ketone;
4) Chlorination reaction: Add cyclohexyl phenyl ketone into the chlorination kettle, raise the temperature to 60±2°C, then add chlorine gas, the reaction is completed, and 1-chlorocyclohexyl phenyl ketone is obtained;
5) Alkaline hydrolysis reaction: add 1-chlorocyclohexyl phenyl ketone into liquid alkali at a weight ratio of 1:0.95-1.10, stir until the reaction is completed, then add toluene for extraction, separate the water phase, and remove Dissolve, recover toluene, and obtain crude 1-hydroxycyclohexylbenzophenone;
6) Refining: Put the crude 1-hydroxycyclohexylbenzophenone into the distillation kettle, distill the product under reduced pressure with a Roots pump, and collect the fractions; put the distilled product into the crystallization kettle , then dissolve and recrystallize with petroleum ether, then centrifuge, distill the mother liquor to evaporate the solvent petroleum ether for reuse, and dry the wet product to obtain the finished product 1-hydroxycyclohexylbenzophenone.
Main reference materials
[1] Concise Photography Dictionary
[2] Illustrated Encyclopedia of Labeling Technology
[3] CN201410495329.X. A kind of ultraviolet curing coating for battery car shell and its preparation method and spraying method Preparation
[4] CN201310005348.5. A UV resin used to produce reflective films and its preparation method
[5] CN201510982397.3. Matte anti-static coating material
[6] CN201810150460.0. Synthesis method of photoinitiator 1-hydroxycyclohexyl phenyl ketone
[7] CN201110200632.9 .Synthesis process of 1-hydroxycyclohexyl phenyl ketone

 微信扫一扫打赏
微信扫一扫打赏

