[Background and Overview][1][2][3]
The formation reaction of carbon-carbon and carbon-heterobonds is a very important type of reaction in organic synthesis. As for the method of constructing carbon-carbon and carbon-heterogeneous bonds, the more commonly used coupling reactions are through functionalized substrates catalyzed by transition metals, such as Heck, Suzuki, Negishi and other coupling reactions. In these reactions, the substrates need to be functionalized in advance, which results in low atom utilization. With the introduction of the concepts of “green chemistry” and “atom economy”, non-functionalized substrates are directly oxidized through carbon-hydrogen oxidation. Coupling reactions to construct carbon-carbon and carbon-heterobonds have aroused great interest among chemists and have achieved great results. In these carbon-hydrogen oxidation reactions, some precious transition metals are generally used as catalysts, such as palladium, which mainly activates sp2-carbon-hydrogen bonds, and uses palladium and substrates to form five- or six-membered palladium rings during the reaction process. structure. Using cheap copper salts or iron salts as catalysts, in the presence of oxidants such as tert-butyl peroxide, tert-butyl peroxide, benzoquinone compounds, etc., a series of sp3 hydrocarbons connected to heteroatoms such as nitrogen and oxygen were studied. Oxidative coupling reactions of bonds. This type of compounds has been studied more in carbon-hydrogen oxidation reactions. The main reason is that most of the biologically active compounds contain heteroatoms. In addition, it may be that these heteroatoms can not only activate the connected sp3 C-H bonds, but also activate the sp3 C-H bonds. Moreover, the intermediate 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) that can be formed stably is a good dehydrogenation oxidant. In the past, it has been used in the aromatization of dihydrobenzene derivatives and the α of sterones. There are many studies on reactions such as β-position dehydrogenation.
2,3-Dichloro-5,6-dicyanobenzoquinone (DDQ) is a strong oxidizing reagent, widely used in the dehydrogenation of compounds, especially for steroidal compounds, with good selectivity. It is an indispensable oxidizing reagent in the current steroid industry. With the deepening of research, it has been gradually discovered that DDQ shows good application prospects in many organic reactions. For example, selective oxidation of alcohols, oxidation of the benzyl position of aromatic rings, carbon-carbon bond formation, carbon heterobond formation, cyclization reactions, protection and deprotection, etc.
[Physical and chemical properties][1]
DDQ is a bright yellow solid with a melting point of 213~215°C. It is easily soluble in ethyl acetate and tetrahydrofuran, and has moderate solubility in dichloromethane, benzene, dioxane and acetic acid. It is insoluble in water, but decomposes in the presence of water to release hydrocyanic acid, so it needs to be stored in a dry state.
[Application][1]
DDQ is increasingly used in organic reactions and is used in more and more fields. The development of new application fields, especially oxidative coupling cyclization reactions, is the main development trend of DDQ in organic reaction application research.
1. Dehydrogenation of carbon-carbon bonds to form double bonds
DDQ is mainly used to dehydrogenate aromatic compounds, heterocyclic compounds, steroid compounds, alcohols and phenols into double bonds. The mechanism is that a negative hydrogen ion of the dehydrogenated product is transferred to DDQ, and then the proton is quickly transferred to hydrogen On the quinone anion, the dehydrogenation product and hydroquinone are obtained. The ability of this reaction to proceed depends on the stability of the carbocation transition state formed after a negative hydrogen ion is transferred from the dehydrogenated product. This reaction is easy to proceed with reactants containing olefins or aromatic hydrocarbons. In the synthesis of melatonin naphthalene derivatives, DDQ was used to react in dichloromethane at room temperature to obtain the intermediate with a yield of 90%:
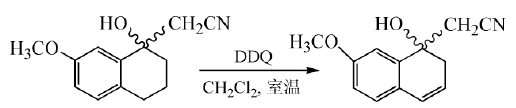
In the final step of the total synthesis of Bauerine C, DDQ is used to dehydrogenate the target product in high yield:
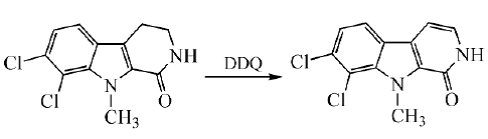
Pyrro[3,4-b]indole was synthesized by dehydrogenation using DDQ in toluene at room temperature:
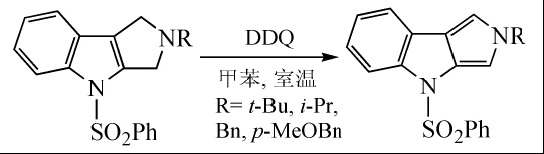
DDQ is also a good aromatization reagent. For carbocyclic compounds with quaternary carbon atoms, when dehydrogenating aromatization with DDQ, the substituents can be displaced without losing carbon atoms. In the synthesis of spinochalcone B, DDQ is used to reflux aromatize cyclohexanone in dioxane to obtain the spinochalcone B intermediate:
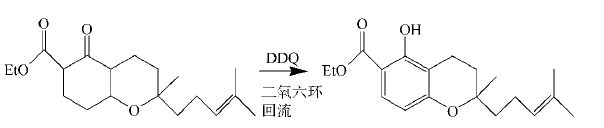
2. Oxidation of benzyl and allyl positions
Generally, saturated alcohols are relatively stable to DDQ, but the hydroxyl groups at the benzyl and allyl positions can be oxidized by DDQ; some secondary alcohols with large steric hindrance can also be oxidized in the presence of reflux, prolonged reaction time and co-oxidant. Can be oxidized to ketones, a process that may be a release of steric tension. Other groups in the benzyl and allyl positions can also be oxidized by DDQ. Use DDQ/PbO2 system to oxidize electron-rich benzyl alcohol to benzophenone:
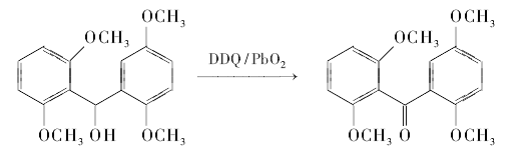
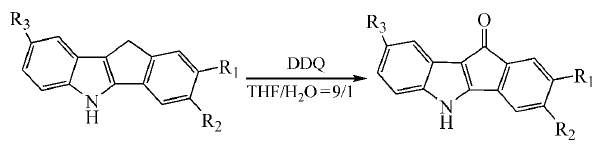
DDQ can oxidize the C-H of benzyl group into carbonyl group under certain conditions. This may be accomplished by first dehydrogenating the benzyl position to form a carbocation, then attacking the carbocation with a hydroxyl or acetoxy anion, and then further oxidizing it to obtain a carbonyl compound. Using tetrahydrofuran/water (9:1) as solvent, use DDQ to oxidize benzylmethylene to carbonyl group at 0℃:
3. Oxidative coupling cyclization
When phenol or enolized ketone is notWhen �� undergoes α, β-dehydrogenation, DDQ catalyzes the formation of the ring. When Sinaiticin is used, DDQ is used to form a pyrone ring in dry dioxane to obtain its intermediate:
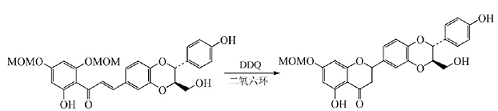
Isoxazole is an important heterocyclic compound. A common synthesis method is to oxidize α,β-unsaturated oxime with iodine/potassium iodide, NBS, etc. Under mild conditions, DDQ is used to oxidize α,β-unsaturated oxime chalcone to obtain 3,5-disubstituted isoxazole, which provides a simple method for the preparation of 3,5-disubstituted isoxazole:
4. Protection and deprotection
DDQ is also a powerful and selective protection and deprotection catalyst. DDQ can gently and efficiently protect sugar hydroxyl groups in dichloromethane system and catalyze the formation of isopropylidene acetal from 2,3-dimethoxypropane (DMP):
5. Other reactions from DDQ’s participation
DDQ can replace some Lewis acids to form C-N bonds under mild conditions, providing an alternative catalyst for compounds that are sensitive to acids. In acetonitrile, use DDQ to catalyze aldehydes and N, N’-diphenylethylenediamine at room temperature to generate 2-substituted 1,3-diphenyl imidazolidine compounds:
Imine salts are highly reactive, so chemists are committed to finding simple synthesis methods. The imine salt can be easily prepared by oxidizing the aminoketene silicon acetal with DDQ, and then the nucleophile is added to the imine moiety to obtain the aminoester in high yield:
[Main reference materials]
[1] Shen Liqun, Lei Fuhou, Huang Suyu. Application progress of 2, 3-dichloro-5, 6-dicyanobenzoquinone in organic reactions [J]. Chemical Bulletin, 2011, 74(6) : 497-507.
[2] You Yecheng, Yu Jiayou. Application of DDQ in the synthesis of β-carboline alkaloids[J]. Synthetic Chemistry, 2000, 8(1): 83-86.
[3] Yuan Kun, Ye Xinyi, Cheng Dongping, et al. Application of DDQ in bimolecular oxidative coupling reaction[J]. Zhejiang Chemical Industry, 2014, 45(8): 21-28.

 微信扫一扫打赏
微信扫一扫打赏

