Background and overview[1][2]
The molecular formula of benzenesulfonic acid is C6H6O3S, with a molecular weight of 158.17. It is colorless and easy to absorb moisture and crystallize. It has a strong irritating effect on skin, eyes and mucous membranes. The melting point is 43~44℃ (containing one and a half molecules of crystal water); the melting point of anhydrous is 50~51℃. Easily soluble in water and ethanol, slightly soluble in benzene, insoluble in ether and carbon disulfide. It is strongly acidic, the acidity is equivalent to that of sulfuric acid, but it has no oxidizing property. The dissociation constant K=0.2 (25℃). The sulfonic acid group of benzenesulfonic acid can be substituted by a variety of groups. It reacts with sodium hydroxide to form sodium phenolate; reacts with sodium cyanide to form benzonitrile; reacts with bromine to form bromobenzene; reacts with nitric acid to form nitrobenzene; Heat with water to remove the sulfonic acid group. Benzenesulfonic acid can be produced by the sulfonation reaction of benzene under the action of concentrated sulfuric acid. It is used to produce phenol and resorcinol, and is also used as a catalyst in esterification reactions and dehydration reactions.
If benzene sulfonic acid is inhaled, please move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if there is eye contact, The eyelids should be separated, rinsed with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately. Advice to protect rescuers is as follows: Move the patient to a safe place, consult a doctor, and if conditions permit, please show this chemical safety data sheet to the doctor who comes to the scene. If there is a small leak, collect the leaked liquid in a sealable container as much as possible, absorb it with sand, activated carbon or other inert materials, and transfer it to a safe place. Do not flush it into the sewer; if there is a large leak, build a dike or dig a pit. Contain, seal the drainage pipe, cover it with foam to inhibit evaporation, use an explosion-proof pump to transfer it to a tanker or a special collector, and recycle or transport it to a waste treatment site for disposal.
Properties and structure
The structure of benzenesulfonic acid is as follows:
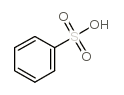
The sulfonic acid group of benzenesulfonic acid can be substituted by a variety of groups. It reacts with sodium hydroxide to form sodium phenolate; reacts with sodium cyanide to form benzonitrile; reacts with bromine to form bromobenzene; reacts with nitric acid to form nitrate Benzene; remove the sulfonic acid group by heat with water:

Preparation[1][2]
Using benzene as raw material, it is obtained by sulfonation with sulfuric acid. 2400L of 93% sulfuric acid is added to the sulfonation pot, and benzene is pumped into the evaporator at a flow rate of 2500-3000L/h. After evaporation and superheating, it becomes superheated benzene vapor with a temperature above 150°C, and reacts in the sulfuric acid layer through the bubbler. , the temperature is around 170°C, and the final free acid is controlled at 4-5%. The reaction is completed in about 10 hours. The unreacted benzene vapor and the generated water vapor are separated by condensation. The acidic benzene is neutralized with liquid alkali and dehydrated with salt before recycling. The benzene-containing benzene sulfonic acid is then passed through a vacuum benzene removal device to remove residual benzene. The sulfonation yield is 96.5%. Neutralize benzenesulfonic acid with sodium sulfite or sodium hydroxide to obtain sodium benzenesulfonate. Neutralization yield 99%. Raw material consumption quota: benzene 600kg/t, oleum 750kg/t.
Apply[2][3]
Benzenesulfonic acid is an important chemical raw material and is mainly used to prepare phenol;���It is used as a catalyst for esterification reaction, dehydration reaction, polymerization reaction and depolymerization reaction in organic synthesis. Application examples are as follows:
1. Used to prepare phenol.
Phenol is an important organic chemical raw material, which can be used to produce phenolic resin, caprolactam, bisphenol A, salicylic acid, picric acid, pentachlorophenol, 2,4-D-adipic acid, phenolphthalein n-acetyl Chemical products and intermediates such as ethoxyaniline have important uses in industries such as chemical raw materials, alkylphenols, synthetic fibers, plastics, synthetic rubber, medicines, pesticides, spices, dyes, coatings and oil refining. The characteristics of the preparation method of phenol are: the raw materials used are benzene and concentrated sulfuric acid, which are obtained by polymerization, distillation, alkali fusion, acidification and crystallization according to a certain ratio; the ratio of the raw materials is that the mass ratio of benzene to concentrated sulfuric acid is 1.5-2.5 ∶1; the concentrated sulfuric acid is 98% concentrated sulfuric acid; the polymerization reaction temperature is 120-140°C, and the reaction time is 30-50 minutes; the distillation temperature is 130-150°C; the alkali fusion is made of benzene Place the sulfonic acid in a container and heat it to 55-65°C. After the benzene sulfonic acid is completely melted, add 2-3 times the mass of benzene sodium hydroxide powder and stir continuously for 1-3 hours to generate sodium phenolate; the acidification is to Sodium phenolate and other mixtures are dissolved in water, and sulfur dioxide gas is introduced under normal temperature and pressure; for crystallization, the solution is cooled to 2-6°C, filtered, and the filter residue is dissolved in distilled water of 65-75°C, and then cooled to 2-6 ℃.
2. Used to prepare 3-chloro-1,2-propanediol. 3-Chloro-1,2-propanediol (referred to as chloroglycerol) is an important glycerol chloride with a wide range of uses: it can be used as an organic synthesis intermediate to synthesize pesticides and dyes.
In the past ten years, applications in aerospace materials, non-ionic contrast agents, electronic chemicals, catalyst synthesis and phase transfer catalyst applications have become increasingly widespread. The purity, impurity content and other indicators of chloroglycerol have been The requirements have also been greatly increased accordingly, so more stringent requirements have been put forward for the industrial production of chloroglycerin. Production method, including the following steps:
(1) Ingredients: Dissolve sulfuric acid, benzenesulfonic acid and succinic acid in deionized water respectively to prepare sulfuric acid solution, benzenesulfonic acid solution and succinic acid solution;
(2) Hydrolysis reaction: First mix part of the sulfuric acid solution, part of the benzene sulfonic acid solution and part of the epichlorohydrin for a period of time, increase the temperature, then add the remaining epichlorohydrin, and proceed in sequence according to time periods Add the remaining benzenesulfonic acid solution and sulfuric acid solution, and then add the succinic acid solution all at once, and continue the reaction by raising the temperature; the amount of epichlorohydrin is the total weight of the sulfuric acid, benzenesulfonic acid and succinic acid aqueous solution. 45~55%.
(3) Neutralization reaction: After the hydrolysis reaction is completed, add alkali solution and stir to make the reaction uniform;
(4) Dehydration and adding alkali: Dehydrate the hydrolyzate after adding alkali under normal pressure first, then continue dehydration when the gas phase temperature is 98~100℃ and the vacuum degree is ≥0.094MPa, then add alkali solution, stirring conditions temperature rise;
(5) Distillation and purification: First heat the material under vacuum conditions, and then transfer it to the distillation system. When the vacuum degree is ≥0.1MPa, the product 3-chloro-1,2-propanediol is obtained by distillation.
Main reference materials
[1] Chemical Dictionary
[2] Li Fen A method for preparing phenol. CN201410571600.3, application date 20141024
[3] Guo Xiangrong; Zhuang Qing; Wang Cui; Guo Yankai; Li Xindong; Zhang Yunling; Wang Xiaoli; Guo Xueyang. A production method of high-purity 3-chloro-1,2-propanediol. CN201010011544.X, application date 20100116

 微信扫一扫打赏
微信扫一扫打赏

