[Background and Overview][1][2][3]
In the past 20 years, hypervalent iodine reagents, as oxidants, have attracted more and more attention from chemists due to their mild reaction conditions, high yield, good selectivity, and environmental friendliness. 1,1,1-triethoxyyl-1,1-dihydroxy-1,2-pheniodoyl-3(1H)-one (DMP, Dess-Martin periodinane) is a A typical hypervalent iodine reagent has been widely used in organic synthesis. DMP can be obtained by heating a mixed solution of 2-iodoacylbenzoic acid (IBX), acetic acid and acetic anhydride. IBX was synthesized in 1893 and has a history of 112 years. However, due to its low solubility in most common organic solvents, there are few reports on its application. In 1994, Frigerio discovered that IBX is easily soluble in DMSO and is very effective in oxidizing alcohols and 1,2-vicinal diols, thus opening a new chapter in the application of IBX in organic synthesis. As an oxidizing agent, the oxidation of primary and secondary alcohols in DMSO shows similar properties to DMP, and when the alcohol has multi-functional groups, IBX has better selectivity than DMP; in addition, DMP is unstable and cannot be stored for a long time, and must be used before use It is very inconvenient to prepare and use. IBX is a cheap, mild oxidant that is easy to prepare and has better selectivity than DMP. It can also be used to prepare lipid and protein metabolism improving agents such as oxandrolone.
【Synthesis】[1]
IBX is a cheap, mild oxidizing agent that is easy to prepare using potassium bromate or Oxone (2KHSO5-KHSO4-K2 SO4) can oxidize o-iodobenzoic acid.
[Application][1]
1. Reaction of IBX and alcohol
1) Reaction of IBX with alcohol in DMSO: The oxidation of hydroxyl group to carbonyl group is a very important transformation reaction in organic synthesis. There are many methods to achieve this transformation under different experimental conditions. In DMSO or DMSO/THF solution, IBX can quickly oxidize primary alcohols and secondary alcohols to obtain aldehydes and ketones respectively at room temperature, while primary alcohols will not be further oxidized to form carboxylic acids, effectively eliminating the formation of by-product carboxylic acids. generate. Under the same conditions, IBX oxidizes 1,2-diol to obtain α-ketool or α-diketone, and the C—C of 1,2-diol The bonds are not cleaved by oxidation. During the reaction process, there is no need to protect the amino group, and it will not destroy furan, pyridine, indole and other heterocyclic rings, silicon ethers, thioethers, allyl groups, alkenes, alkynes, acetals, thioketals, ester groups, Functional groups such as amide groups are not affected during the reaction:
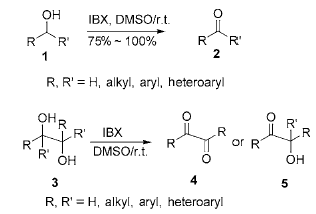
Benzyl, allyl, propargyl alcohol and diol are oxidized by IBX in the presence of stable Wittig reagent to obtain α, β -Unsaturated esters. This method is particularly useful if the intermediate aldehyde is unstable or difficult to separate:

The oxidation reaction of alcohol by IBX is usually carried out in DMSO or DMSO/THF solution. Simply heat (80 ℃) alcohol, IBX and organic solvents such as ethyl acetate, chloroform, benzene, acetonitrile, acetone, dichloro A mixture of methane, etc. can oxidize primary and secondary alcohols into the corresponding aldehydes and ketones; after the reaction is completed, the insoluble by-products and solvents are filtered to obtain the corresponding carbonyl compounds, with a yield of 90% to 100%.
2) Reaction of IBX with alcohol under solvent-free conditions: Under solvent-free conditions, IBX reacts with primary alcohol (1.25:1, material ratio) at 60-70°C to obtain the corresponding aldehyde, and the yield can be Reaching 72%~95%. If there is a large excess of IBX or the reaction is performed at a temperature higher than 90°C, some aldehydes will be oxidized into carboxylic acids. Under the same conditions, the secondary alcohol is oxidized to obtain the corresponding ketone, and the yield is also relatively high. However, under solvent-free conditions, IBX is limited to oxidizing allyl alcohol and benzyl alcohol, and aliphatic alcohols do not react. In addition, it is only suitable for small-dose reactions and is suitable
Used for large-scale synthesis reactions; if the reaction temperature is relatively high, there is a risk of explosion.
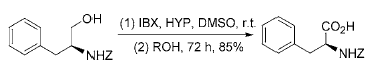
3) Reaction in which IBX directly oxidizes primary alcohol to obtain carboxylic acid: Use IBX to oxidize primary alcohol in DMSO to obtain phase
The aldehyde will not be further oxidized to carboxylic acid. However, in the presence of O-nucleophiles such as 2-hydroxypyridine (HYP), N-hydroxysuccinimide (NHS) or 1-hydroxybenzotriazole (HOBt), IBX Primary alcohols can be directly oxidized to carboxylic acids at room temperature, with a yield of 64% to 95%. This reaction can be used to directly oxidize N-protected β-amino alcohols to the corresponding amino acids without racemization:
2. Reaction of IBX with nitrogen-containing organic compounds
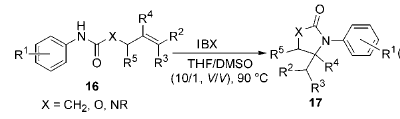
1) Application of IBX in ring-closing reactions of unsaturated N-aryl amides, carbamates, and ureas: methods for constructing C—N bonds are: through N-nucleophilicity Substitution of O-functional groups by reagents, rearrangement of carbonyl functional groups, and reductive amination, etc. However, there are few methods to directly connect N to C without O connection without producing harmless by-products. It is obtained by reacting IBX with unsaturatedN-arylamide, carbamate, and urea. Various nitrogen-containing five-membered heterocyclic compounds use this method to selectively connect the N-functional group to the unactivated olefinic bond to form
Into a new C-N bond. This is useful in the synthesis of γ-lactams and cyclic amino groups.It has clever applications in important structural units and intermediates such as acid esters and amino sugars:
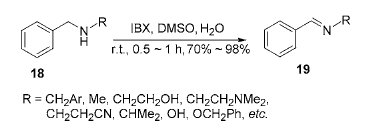
2) Application of IBX in amine oxidative dehydrogenation reaction: Oxidizing amines to imines is a very useful transformation, and there are many related literature reports, but each method has some major shortcomings. The lack of a mild and versatile method for the oxidation of amines in organic synthesis is surprising because structural units such as imines and oximes can be easily prepared through the oxidation of amines, and these structural units are found in many heterocyclic rings. It has important uses in the synthesis of compounds. For this reason, the reaction between IBX and benzylamine was studied, and it was found that IBX can oxidize secondary amines into imines under very mild conditions. The reaction time is short, the yield is high, and it is selective.
3) Application of IBX in the aromatization reaction of nitrogen-containing heterocyclic compounds: Many biologically active natural products and drugs are nitrogen-containing heterocyclic compounds, so the synthesis methods of nitrogen-containing aromatic heterocyclic compounds are subject to Chemists are paying widespread attention. IBX can be used to synthesize substituted aromatic heterocyclic compounds such as imidazole, isoquinoline, pyridine and pyrrole from cyclic amines:
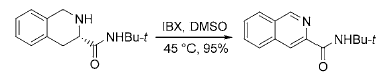
3. Reaction of IBX with sulfur-containing organic compounds
1) IBX oxidizes thioether to sulfoxide: it is a very useful compound that can be obtained through a series of reactions
To convert sulfoxides into many organosulfides, these transformations are useful in the synthesis of drugs and sulfur natural products. To oxidize thioether to sulfoxide, the reaction conditions usually need to be strictly controlled, including controlling the oxidant
dosage to reduce the formation of sulfone by-products. Oxidation of thioethers using IBX in the presence of catalytic amounts of tetraethylammonium bromide (TEAB) afforded sulfoxides in almost quantitative yields, with no sulfone formation observed. If TEAB is not added, the reaction is slower and takes 12 to 36 hours.
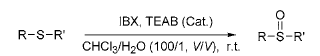
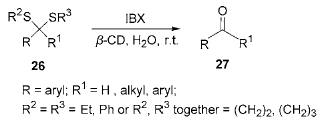
2) Application of IBX in thioacetal (ketone) deprotection reaction: Converting carbonyl group into thioketal (aldehyde) is a common method to protect carbonyl group. However, it is more difficult to protect them. The usual methods
The method requires the use of harsh oxidizing conditions or mercury salts, so it is necessary to find a milder reagent with less toxicity. Using acetone-water (2∶15,V:V) as the solvent, in β-cyclodextrin (β Under the catalysis of em>-CD), IBX is used to hydrolyze thioketal (aldehyde) into the corresponding carbonyl compound. The reaction can be carried out at room temperature, and the yield is as high as 85% to 94%. And halogen atoms, nitroso groups, hydroxyl groups, alkoxy groups, conjugated double bonds, etc. are not affected during the reaction.
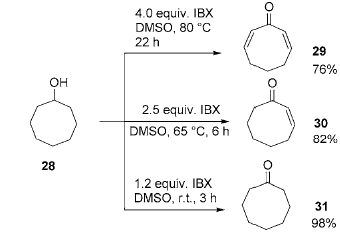
4. IBX oxidation of aldehydes and ketones to prepare α, β-unsaturated carbonyl compounds
In organic chemistry, α, β-unsaturated carbonyl compounds are a common and useful class of compounds, but their synthesis is sometimes a complicated and complicated task. Difficult work. There have been many reports on their synthesis methods in the past. A common method is to first prepare enol silyl ethers from carbonyl compounds, and then use palladium-catalyzed oxidation to obtain α, β-unsaturated carbonyl compounds. Another method is to use selenium reagents and prepare them through a one- or two-step reaction. By adjusting the amount of IBX, reaction temperature and reaction time, products with different saturations can be obtained:
5. Application of IBX in the preparation of lactones
Lactones can be prepared by oxidizing lactones. Lactoacetal is insoluble or slightly soluble in most organic solvents, but is soluble in DMSO. Using DMSO as the solvent, use IBX at room temperature in the next step
1,4-diol is oxidized to obtainγ-lactoacetal with a yield of 60% to 88%, but no lactone is produced. They think it may be due to large steric hindrance. . Using ethyl acetate/DMSO (9:1,V:V) as the solvent and heating to reflux, IBX oxidized the lactone to obtain the lactone with a yield of up to 66 %~91%.
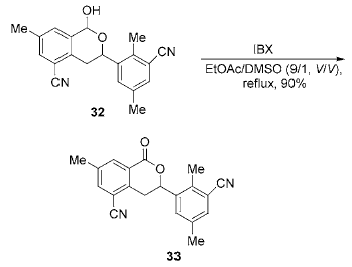
6. IBX oxidizes the carbon atoms connected to the aromatic ring
The C atom connected to the aromatic ring is an electron-rich site and can be oxidized to a carbonyl group by IBX. This method can be used to construct a carbonyl group at the position connecting the aromatic ring. Dissolve the reaction substrate and IBX in fluorobenzene/DMSO (2:1,V:V) or pure DMSO, heat to 80~90 °C, and react. It can be carried out with high yield and few by-products.
7. Other examples of successful applications of IBX in organic synthesis
α-hydroxyketone and α-aminoketone are important synthetic intermediates in organic and medicinal chemistry. In the presence of β-cyclodextrin and using water as the solvent, IBX oxidizes the epoxy compound and aziridine to obtain α-hydroxyketone and α-Aminoketone. This is the first example of the one-step synthesis of α-aminoketone from aziridine:
α-functionalized ketones are very useful intermediates in the synthesis of many heterocyclic compounds, natural products and related compounds. The compound o-iodine was synthesized using IBX in the presence of potassium iodide. Benzoic acid-1-o-iodobenzoyloxy-2-oxoarylethyl ester:
[Main reference materials]
[1] Qin Kaiyun, et al. Application of 2-Iodoxybenzoic acid (IBX) in organic synthesis. 2006. PhD Thesis.
[2] He Shihua. Application of 2-iodoacylbenzoic acid in the synthesis of oxandrolone. Chinese Journal of Pharmaceutical Industry, 2008, 39.8: 573-575.
[3] Liu Yujiao. Research on the chemical components of pork kidney beans and cloves and the application of IBX in the transformation of plant components. 2015. Master’s Thesis. Yunnan Normal University.
It is a very useful intermediate in the synthesis of chemicals, natural products and related compounds. The compound o-iodobenzoic acid-1-o-iodobenzoyloxy-2-oxoarylethyl was synthesized using IBX in the presence of potassium iodide. Base ester:
[Main reference materials]
[1] Qin Kaiyun, et al. Application of 2-Iodoxybenzoic acid (IBX) in organic synthesis. 2006. PhD Thesis.
[2] He Shihua. Application of 2-iodoacylbenzoic acid in the synthesis of oxandrolone. Chinese Journal of Pharmaceutical Industry, 2008, 39.8: 573-575.
[3] Liu Yujiao. Research on the chemical components of pork kidney beans and cloves and the application of IBX in the transformation of plant components. 2015. Master’s Thesis. Yunnan Normal University.

 微信扫一扫打赏
微信扫一扫打赏

