Background and overview[1-2]
BenzoBüfuran-3-acetonitrile is a pharmaceutical intermediate. It has been reported in the literature that it can be prepared from diethyl cyanomethyl phosphate and 3-coumaranone through a one-step reaction. There are reports in the literature that it can be used to prepare compounds that modulate the activity of nuclear receptors.
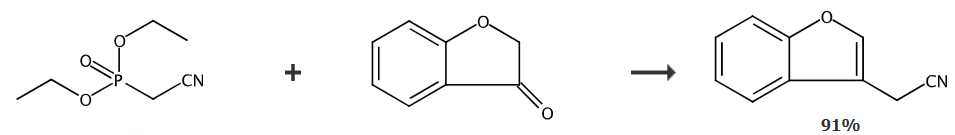
Preparation[1]
Mix sodium hydride (268 mg, 6.7 mmol) in anhydrous THF (10 ml) under a nitrogen atmosphere at 0°C to make a slurry, and then add diethyl cyanomethyl phosphate (1.1 ml, 6.7 mmol) dropwise. , and then stir the entire system for 45 minutes. Then 3-coumaranone (Lancaster) (900 mg, 6.7 mmol) was added dropwise, and the entire system was stirred at room temperature for 45 minutes. The reaction was diluted with EtOAc (15 ml) and water (15 ml), then the organic layer was separated, dried (MgSO4) and evaporated, and the residue was subjected to pentane using 0-5% EtOAc Flash chromatography of the solution gave the title product (940 mg, 91%); 1HNMR (400MHz, CDCl 3), δ: 3.77 (s, 2H), 7.25 -7.40 (m, 2H), 7.52 (dd, 1H), 7.58 (dd, 1H), 7.67 (s, 1H); LRMS: M+NH4+, 175. (TS+).
Apply[2]
CN201110243983.8 reports that benzoBü furan-3-acetonitrile can be used to prepare compounds that modulate nuclear receptor activity with the following structure. This class of compounds has very high affinity for farnesine X receptors. Unexpectedly, this class of compounds has the power to simultaneously reduce plasma triglyceride and cholesterol levels in normal and hyperlipidemic animal models.
References
[1][China invention, China invention authorization] CN02805409.1 N-phenylpropylcyclopentyl-substituted glutaramide derivative as NEP inhibitor of FSAD
[2][Invented in China] CN201110243983.8 Azazoindole derivatives as drugs

 微信扫一扫打赏
微信扫一扫打赏

