Introduction[1]
Oxalic acid and oxalate esters are important organic chemical raw materials and are widely used in the preparation of various dyes, medicines, important solvents, extractants and various intermediates. Dibenzyl oxalate is usually white flaky crystals, insoluble in water, has little coloration, low melting point and stable chemical properties. It is an important organic synthesis intermediate.
Usually produced by the reaction of oxalic acid dichloride and benzyl alcohol, with a yield of 46%. However, this process has long reaction time, high energy consumption and low yield. Microwave radiation is also used to synthesize dibenzyl oxalate. Although the reaction time is short, the yield is not high at 62.96%, which is not conducive to industrial production.

Preparation method[2]
Method 1:
Add experimental amounts of oxalic acid, benzyl alcohol, and water-carrying agent into a 100mL three-necked flask equipped with a thermometer, water separator, and reflux condenser, heat and stir until the oxalic acid is completely dissolved; then add a certain amount of catalyst, and heat and reflux until divided No water beads are formed in the water vessel, indicating that the reaction is complete. Then change the reactor to a distillation device, evaporate most of the water-carrying agent, and pour the remaining reaction solution into 100g of crushed ice while hot, and a large amount of white precipitate will form. Leave to stand until complete precipitation, filter with suction, wash the filter cake twice with distilled water, and drain. Take out the filter cake, recrystallize it with 50 mL of ethanol, and white flaky crystals will precipitate. Filter it with suction, dry it in the air, weigh it, and calculate the yield of dibenzyl oxalate.
Method 2:
The synthesis mechanism of dibenzyl oxalate is shown in Figure 1. Add oxalic acid dihydrate 7.69 (0.06m01) into a three-necked flask, cool it in an ice bath, slowly add 20mL of triethylamine (0.14m01) dropwise while stirring, complete the dripping, remove the ice bath, and stir magnetically at room temperature for 2 hours. Solution A. Slowly add 16.8 mL (0.144 m01) of benzyl chloride into solution A while stirring, and react in a water bath at 80°C for 2 hours. Cool, let stand until complete precipitation, filter with suction, wash the filter cake twice with distilled water, and drain. Take out the filter cake and recrystallize it with 50raL ethanol, and white flaky crystals will precipitate. Filter, wash, dry, weigh, and calculate the yield.
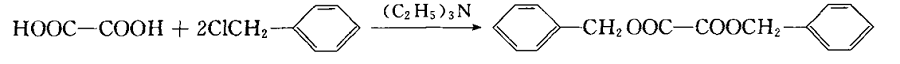
Method 3:
In a three-necked flask equipped with a thermometer and a reflux condenser, add sodium oxalate (2.68g, 0.02mol) and a certain volume of water, heat to dissolve, add a certain amount of benzyl bromide, TBAB, 90°C React for 6 to 10 hours. Cool, let it fully crystallize, filter with suction, wash the filter cake twice with 20 mL of ice water, dry, weigh, and calculate the reaction yield of dibenzyl oxalate. The synthesis method is as follows:
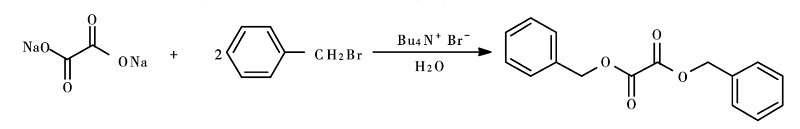
Main reference materials
[1] Li Pigao, & Liu Dongping. (2007). Synthesis of dibenzyl oxalate by microwave radiation. Fine Petrochemicals (02), 28-30.
[2] Yan Tao, Zhang Zaiqing, Sun Xuejun, & You Jinmao. (2009). Synthesis of dibenzyl oxalate by catalyst%. Journal of Qufu Normal University (Natural Science Edition), 035(003) ), 61-63.
[3] You Jinmao. (2009). Catalytic synthesis of dibenzyl oxalate. Journal of Qufu Normal University, 35(3).

 微信扫一扫打赏
微信扫一扫打赏

