Background and overview[1]
1-(4-Aminobenzene)-1,2,2-tristyrene can be used as a synthetic intermediate, such as the preparation of tetraphenylethene (TPE) compounds. Tetraphenylethene compounds have large In a conjugated system, the four benzene rings of the molecule are connected to the same isolated double bond. Due to the existence of steric hindrance, the benzene rings are twisted with each other, so the molecule takes on the shape of a propeller. This type of compound has excellent luminescent properties and is easy to synthesize. It is an excellent aggregation-induced emission (AIE) compound and has been intensively studied in the fields of organic light-emitting diodes, liquid crystal devices, supramolecular assembly, drug release, sensors and other fields.
Preparation[1]
1-(4-Aminobenzene)-1,2,2-tristyrene is synthesized as follows:
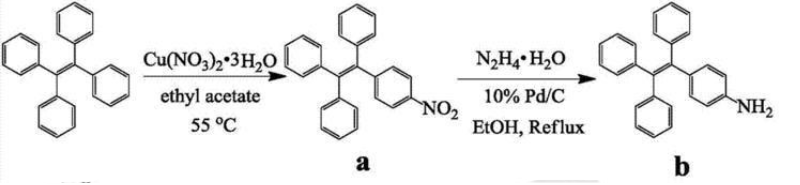
1) Synthesis of compound a
Dissolve tetrastyrene (3.32g, 10mmol) in ethyl acetate (100ml). Add Cu(NO3)2·3H2O (3.38g, 14.0mmol) and acetic anhydride (2.68ml, 28.2mmol). The mixture was stirred at 55°C for 10 hours, cooled to room temperature, poured into water, and extracted with ethyl acetate. The upper organic phase was dried with MgSO4, filtered and concentrated. Then it was purified and concentrated by silica gel flash column chromatography to obtain compound a. Yield: 3.24g, 86%. 1HNMR (400MHz, DMSO): δ8.02(2H,d,J=8.7Hz),7.23–7.12(11H,m),7.00(6H,dt,J=3.7,2.1Hz ).13CNMR(101MHz,DMSO):δ151.04,146.05,142.94,139.08–138.16,132.39,127.84,123.55.GC/MS:m/z377[M]+.
2) Synthesis of 1-(4-aminobenzene)-1,2,2-tristyrene
Compound a (3.77g, 10mmol) was dissolved in ethanol (100ml). 10% Pd/C (1g) and hydrazine monohydrate (6.00g, 120mmol) were added to the above solution. The mixture was refluxed for 5 hours, then the solid Pd/C was filtered and the solvent was removed in vacuo. The residue was purified by silica gel flash column chromatography to obtain 1-(4-aminobenzene)-1,2,2-tristyrene. Yield: 3.54 g, 94%. 1HNMR (400MHz, DMSO): δ7.19–6.89 (17H, m), 6.58 (2H, d, J = 8.4Hz), 6.28 (2H, d, J = 8.4Hz). 13CNMR(101MHz,DMSO): δ147.77,145.23–144.04,141.65,138.32,132.42–130.48,126.61,113.57.GC/MS:m/z347[M]+.
References
[1]CN201810131441.3 A tetrastyrylthiazole solvent water fluorescent probe and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

