Background[1][2]
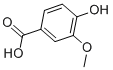
The vanillic acid system name is “4-hydroxy-3-methoxybenzoic acid”. Molecular formula C8H8O4. Molecular weight 168.14. White needle-like crystals. Odorless, sublimates and does not decompose. Melting point 210℃. Easily soluble in ethanol, soluble in ether, slightly soluble in water. It does not develop color when reacting with ferric chloride. Vanillic acid is one of the active ingredients of Huahuanglian. The structures of Coptidis glycosides contain vanillic acid group, ferulic acid group and cinnamoyl group respectively. After hydrolysis, they are vanillic acid, ferulic acid and cinnamic acid. Vanillic acid is one of the antibacterial components of Coptidis Coptidis. The content of it was measured. The amount of vanillic acid can be used as an indicator to measure the quality of Coptis chinensis.
Vanillic acid currently on the market is mainly derived from plant extraction, biological fermentation synthesis, chemical synthesis, etc. The plant extraction method has high raw material costs and requires cultivating a large number of plants. It is greatly affected by weather and region, and cannot meet market demand at all. The process of biosynthesizing vanillic acid is limited by substrates, strains and enzymes, and the conversion rate is not high. The production process has not been widely used. The chemical synthesis process of vanillic acid mainly uses vanillin as raw material and is prepared by oxidation (silver oxide) or alkali fusion (potassium hydroxide, below 240°C). It is also partially removed from 3,4-dimethoxybenzoic acid. Based on the technology.
The oxidation method faces disadvantages such as high cost and large pollution, and is not suitable for large-scale production. The alkali fusion process has problems such as violent reactions, poor safety, low productivity, and is not widely used in industry. As a raw material, 3,4-dimethoxybenzoic acid is more expensive than vanillin and is not suitable for large-scale production. To sum up, the current vanillic acid synthesis process has poor safety, low productivity, low yield, complex operation, and poor yield and purity.
Structure
Apply[2][3][4][5]
Vanillic acid can be mainly used in the synthesis of spices or medicines, such as 5-nitrovanillic acid, which can be used in 3,5-disubstituted catechol catechol-O-methyltransferase (COMT-I) The synthesis of inhibitor tolcapone, or as a precursor for the biosynthesis of rifamycin antibiotics (3-hydroxymethoxyrifamycin, 3-hydroxyrifamycin).
As one of the active ingredients of vanilla beans, vanilla pods, Coptidis chinensis and other plants, vanillic acid is used at home and abroad for its antibacterial, anti-inflammatory, anti-oxidation, inhibition of tyrosinase activity, allelopathic effects, nerve regulation, and promotion of There has been some research on coagulation activity, and it has good activity and good market prospects. Examples of its application are as follows:
For the synthesis of vanillin. Vanillin, also known as vanillin, has a chemical name of 3-methoxy-4-hydro xybenzaldehyde. It is a broad-spectrum high-end spice, known as the king of spices, and is widely used in food, beverages, spices, medicine and other fields. The annual consumption is very large, and most of the products are chemically synthesized. Pure natural products are mainly extracted from vanilla pods, and the output is very small, which is far from meeting the market demand.
Natur-identical (NI) vanillin produced by microbial transformation of natural precursors is called “biovanillin” and can replace natural vanillin. The specific method is to screen out a strain of Pycnoporus vermilion SW-0203 that can transform vanillic acid into vanillin. After optimizing the culture medium and transformation conditions, it can convert 1.682 g/L vanillic acid into 0.875 g/L vanillin. Aldehyde, molar conversion rate is 57.5%. A scale-up test was conducted on a 25 L tank, and the vanillin yield was 0.818 g/L, and the molar conversion rate was 53.7%. The product in the conversion solution was extracted to obtain vanillin crystals with a purity of 95% and an extraction yield of 63.9%.
In addition, some studies have used enzyme kinetics methods to study the inhibitory effect of vanillic acid on the activities of tyrosinase monophenolase and diphenolase. The results show that vanillic acid has an inhibitory effect on tyrosinase monophenolase and diphenolase. The vanillic acid concentration (IC50) that causes the activity of monophenolase and diphenolase to decrease by 50% is approximately 1. 3 mmol /L and 2. 6 mmol /L respectively.
Vanillic acid can significantly prolong the lag time of monophenolase. 2 mmol/L vanillic acid can extend the lag time from 1.1 min to 4.7 min. The inhibitory effect of vanillic acid on diphenolase is mixed inhibition and inhibition of free enzyme.The constant (KI) and the inhibition constant (KIS) of the enzyme-substrate complex are 1. 76 mmol /L and 8. 57 mmol /L respectively.
In addition, there are also studies exploring the autotoxic effects of the phenolic acid autotoxic substance vanillic acid, studying its effects on peanut seed germination and seedling growth, and revealing the response of rhizosphere soil microorganisms to autotoxic substances during the peanut growth period. law. Taking the peanut variety Fuhua No. 12 150GY as the test material, the petri dish culture test set up 6 treatments: 0, 0.01, 0.03, 0.05, 0.07, 0.09 mmol·L-1 vanillic acid solution; the nutrient bowl planting test set up 5 treatments: 0, 0.01, 0.03, 0.05, 0.07 mmol·L-1 vanillic acid solution; the pot experiment set up 5 treatments: the dosage of vanillic acid was 0, 0.01, 0.03, 0.05, 0.07mg·kg-1 dry soil.
Respectively study the effects of exogenous addition of vanillic acid on peanut seed germination, seedling growth and rhizosphere microflora. The results show:
(1) After being treated with vanillic acid solutions of different concentrations, the germination rate, germination potential and germination index of peanut seeds were all lower than CK, and were significantly different from the control. When the concentration of vanillic acid solution was 0.09 mmol·L-1, the germination rate, germination potential and germination index were reduced by 39%, 66.3% and 55.9% respectively compared with CK, and the autotoxic effect response index reached the maximum value.
(2) After being treated with vanillic acid solutions of different concentrations, the main root length, dry weight of each plant, chlorophyll content, net photosynthetic rate and stomatal conductance of peanut seedlings were all lower than CK, and there were significant differences from the control. When vanilla When the acid solution concentration was 0.07 mmol·L-1, each index was reduced by 37.3%, 40.0%, 19.0%, 53.9% and 49.1% respectively compared with CK, and the autotoxic effect response index reached the maximum value. The changing trend of intercellular CO2 concentration is opposite to the above indicators. It shows an upward trend with the increase of vanillic acid concentration. When the concentration of vanillic acid solution is 0.07 mmol·L-1, the intercellular CO2 concentration increases by 46.1% compared with the control.
(3) When the vanillic acid concentration is ≥0.03 mmol·L-1, the total absorption area, active absorption area and root activity (active absorption area/total absorption area) of peanut roots are lower than CK, and the MDA content of leaves is higher than the control , were significantly different from the control. When the concentration of vanillic acid solution was 0.07 mmol·L-1, each index decreased by 22.4%, 54.2% and 40.6% respectively compared with the control, and the MDA content increased by 43.3%.
(4) The number of actinomycetes in the rhizosphere decreased significantly with the increase of oxalic acid concentration in the early growth stage of peanut, but the difference between treatments was not significant after entering the pod-bearing stage. There was no significant difference in the number of rhizosphere bacteria among the treatments in the early stages of peanut growth, but it significantly decreased with the increase of vanillic acid concentration after entering the pod-setting stage. High concentration of vanillic acid (0.07 mg·kg-1 dry soil) has an inhibitory effect on the growth of rhizosphere fungi, while low concentration of vanillic acid (0.01 mg·kg-1 dry soil) has a promoting effect on the growth of rhizosphere fungi.
Therefore, vanillic acid has a certain inhibitory effect on peanut seed germination and seedling growth. Vanillic acid will also inhibit the photosynthesis of peanut seedlings, reduce root activity, and promote the production of malondialdehyde in the seedling leaves. In addition, vanillic acid solutions of different concentrations will reduce the number of bacteria and actinomycetes in the peanut rhizosphere, inhibit the growth and reproduction of bacteria and actinomycetes in the rhizosphere soil, and have a low-promoting and high-inhibiting effect on soil fungi. , that is, low-concentration vanillic acid solutions promote the growth of fungi in peanut rhizosphere soil; while high-concentration solutions have a certain inhibitory effect on fungal growth.
Preparation[6]
Add a solution of 170g of silver nitrate dissolved in 1L of water into a 2L beaker, and treat with a solution of 44g of 97% sodium hydroxide dissolved in 400ml of water while stirring. The mixture was stirred for 5 min, filtered to remove silver oxide, and washed with water to remove nitrates. Move this silver oxide to a 4L beaker, add 2L of water, and treat with 200g of sodium hydroxide solid under vigorous stirring. Heat the mixture to 55-60°C. Continue stirring and add 152g vanillin. Silver oxide changes to metallic silver and releases a lot of heat. Continue stirring for 10 minutes and filter. Wash the precipitated silver with 100 ml hot water. Combine the filtrate and washing liquid, and quickly introduce sulfur dioxide for 2 minutes. Pour the resulting solution into 1.1L hydrochloric acid (1:1) under vigorous stirring, cool to 15-20°C, filter, and wash with ice water. After being drained, the yield is 140-160g and the yield is 83-95%.
Main reference materials
[1] Synthetic fragrance product technical manual
[2] CN201820021553.9 A kind of vanillic acid continuous production equipment
[3] Wang Mingjun, Zheng Pu, Sun Zhihao, et al. Microbial conversion of vanillic acid to produce vanillin [J]. Food and Fermentation Industry, 2004, 30(2): 43.
[4] Gong Shengzhao, Yang Zhuoru, Lin Xi. Inhibitory effect of vanillic acid on catalytic activity of tyrosinase [J]. Fine Chemicals, 2005, 22(12): 927-930.
[5] Huang Yuqian, Yang Jinfeng, Liang Chunhao, et al. Effects of vanillic acid on peanut seed germination, seedling growth and rhizosphere microflora [J]. Chinese Agricultural Sciences, 2018, 51(9): 1735-1745 .
[6] Duan Changqiang et al., Handbook of Modern Chemical Reagents (1), Chemical Industry Press, 507-508 (1988)

 微信扫一扫打赏
微信扫一扫打赏

