Background and overview[1]
4-Bromo-2-iodophenol, also called 2-iodo-4-bromophenol, is an organic intermediate that can be prepared by nitration and reduction of p-bromophenol, followed by diazotization and iodine substitution.
Preparation[1]
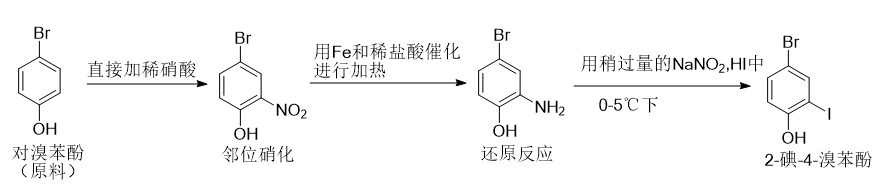
Synthesis of 2-nitro-4-bromophenol:
Add p-bromophenol to the chemical reaction kettle, then directly add dilute nitric acid at room temperature, let it stand for 20 minutes, fractionate the reactants under reduced pressure, and collect the 125°C, 2.35kPa fraction to obtain 2-nitro-4 -Bromophenol;
Synthesis of 2-amino-4-bromophenol:
Place 2-nitro-4-bromophenol in a chemical reaction kettle, add Fe and dilute HCL to 2-nitro-4-bromophenol, and heat to achieve ortho-nitration, and keep the temperature at 70 ℃, continue for 1 hour, carry out vacuum fractionation of the reactant, collect the 105 ℃, 2.57kPa fraction to obtain 2-amino-4-bromophenol;
Synthesis of 4-bromo-2-iodophenol:
Add a slight excess of NaNO2,HI to the reaction kettle, then add 2-amino-4-bromophenol, keep the temperature at 0°C, and perform a reduction reaction for 40 minutes. The reactants were fractionated under reduced pressure, and the 120°C, 3.45kPa fraction was collected to obtain 4-bromo-2-iodophenol.
Apply[1]
4-Bromo-2-iodophenol can be used to prepare 2,5-dibromoiodobenzene. 2,5-Dibromoiodobenzene is a very important fine chemical raw material. It is a halogenated compound of benzene and a white solid. It is mainly used in the synthesis of medicines, pesticides, dyes, plastics and functional polymer materials.
Synthesis of 2,5-dibromoiodobenzene: Place 4-bromo-2-iodophenol in a chemical reaction kettle, then add dioxane solvent, and stir at room temperature. During this process, gradually Add phosphorus tribromide, fractionate the reactant under reduced pressure, and collect the 135°C, 3.35kPa fraction to obtain 2,5-dibromoiodobenzene.
References
[1] [Chinese invention] CN201810970282.6 A synthesis method of 2,5-dibromoiodobenzene

 微信扫一扫打赏
微信扫一扫打赏

