Background and overview[2-3]
2-Methyl-3-nitrobenzoic acid can be used as an intermediate of methoxyfenozide. Methoxyfenozide is a new type of specific phenylhydrazide low-toxicity insecticide. It has highly selective insecticidal activity against lepidopteran pests, with a mainly contact effect and a certain systemic effect. 2-Methyl-3-nitrobenzoic acid can also be used as a starting material for lenalidomide. Lenalidomide is used to treat myelodysplastic syndrome. It is a new immune-modulating, non-chemotherapy anti-cancer drug. It is an upgraded drug of thalidomide. It affects a variety of biological pathways in cells. It is a new type of immunomodulator developed by the American company Celgene. It was approved by the FDA for marketing in January 2006. The trade name is Revlimid. It is mainly used to treat myelodysplastic syndrome (myelodysplastic syndrome) with 5q deletion (the gap gene deletion on the long arm of the fifth pair of chromosomes). MDS) subtypes and multiple myeloma (MM).
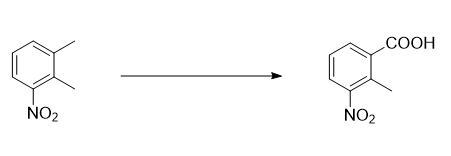
Preparation[1-2]
Report 1,
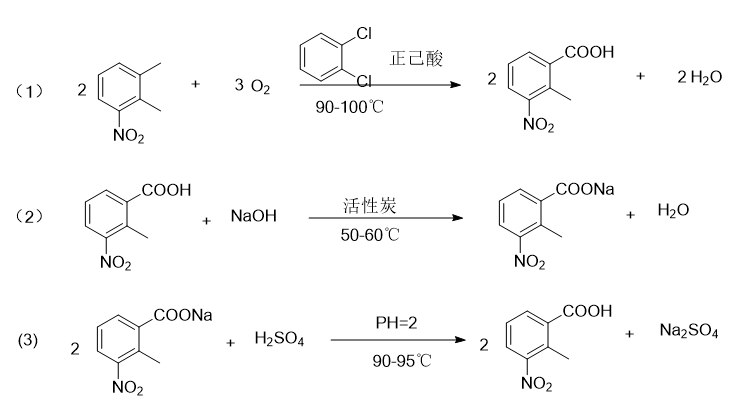
Put 200g 3-nitro-o-xylene, 700g o-dichlorobenzene, 200g n-hexanoic acid, 12g cobalt acetate, 12g manganese acetate, 6g tetrabromoethane into a 1000ml four-neck bottle with reflux condenser and water separator. , add oxygen to maintain 1.2L/min, raise the temperature to 90°C, and keep the reaction at 90-100°C for 18 hours. Approximately 23g of water will be separated. During sampling, 3-nitro-o-xylene ≤ 1% is the end point of the reaction. If the raw material If there is more, continue to react with oxygen until the reaction reaches the end point; cool and filter to obtain 235g of crude product (containing solvent); the mother liquor is used as the next batch of solvent and a certain amount is added before use, and a catalyst of 5% of the original input amount is added, and 3-nitrate is added The o-xylene was reacted with oxygen again, and the result was the same as the original batch.
Put 235g of crude product, 1400ml of water, and 45g of caustic soda with a purity of 99% into a 2000ml four-neck bottle, raise the temperature to 50-60°C, react for 30 minutes, place it in a separatory funnel, and separate out 30g of oil (for the next batch of reactions) (for solvent), return the aqueous solution to the 2000ml four-neck bottle, add 6g of activated carbon, stir and decolorize at 50-60°C for 40 minutes, filter, heat the mother liquor to 90°C, add dilute sulfuric acid dropwise at 90-95°C to adjust pH=2. React for 30 minutes, cool to 30°C, filter, wash with water and dry to obtain 195g of finished product, mp: 182-184°C. The 2-methyl-3-nitrobenzoic acid content is 98.5% as measured by high performance liquid chromatography (HPLC). The rate is 80%.
Report 2,
In a 500ml three-necked flask, add 20g 3-nitro-o-xylene (0.132mol), 0.164g cobalt acetate (0.00066mol), then add 76g n-hexanoic acid (0.66mol), and slowly add 9.87g (0.29mol) dropwise ) hydrogen peroxide, slowly increase the temperature to 60 degrees Celsius, and keep the reaction for 5 hours. HPLC detects the reaction. When the raw material 3-nitro-o-xylene remains less than 2%, add 34g (0.85mol) sodium hydroxide aqueous solution to the reaction system and separate the liquids. , use 32.8g (0.90mol) hydrochloric acid to adjust the pH value of the water layer to about 2, and then suction filtrate to obtain 19.11g of the 2-methyl-3-nitrobenzoic acid product, with a yield of 80%.
Apply[3]
CN201410579059.0 discloses a method for preparing lenalidomide. First, 2-methyl-3-nitrobenzoic acid is esterified to obtain methyl 2-methyl-3-nitrobenzoate, and then After bromination, 2-bromomethyl-3-nitrobenzoic acid methyl ester is obtained; then L-glutamine is reacted with di-tert-butyl carbonate to obtain N-Boc glutamic acid; N-Boc-glutamic acid is obtained in In the presence of a condensing agent and a catalyst, Boc-protected 3-amino-2,6-piperidinedione is obtained, and then reacted with acid to obtain 3-amino-2,6-piperidinedione hydrochloride; -2,6-Piperidinedione reacts with 2-bromomethyl-3-nitrobenzoic acid methyl ester to obtain 3-(4-nitro-1,3dihydro-1-oxo-2hydro-isoindole Indo-2-yl)piperidine-2,6-dione; final reduction gives lenalidomide. The product yield obtained by the method of the invention is high.
References
[1] CN201510492435.7 New preparation method of 2-methyl-3-nitrobenzoic acid
[2] [Chinese invention] CN201711183025.X A clean production method for oxidizing 3-nitro-o-xylene to 2-methyl-3-nitrobenzoic acid
[3] [Chinese invention] CN201410579059.0 A method of preparing lenalidomide

 微信扫一扫打赏
微信扫一扫打赏

