Background and overview[1]
4,4’-Bis(2-bromoacetyl)biphenyl is a key intermediate in the synthesis of the anti-hepatitis C drug daclatasvir. Daclatasvir is a new hepatitis C drug developed by Bristol-Myers Squibb in the United States. On July 24, 2015, the U.S. FDA approved it for the treatment of chronic hepatitis C. The trade name is Daklinza. It is an NS5A inhibitor and is suitable for gene therapy. 1, 2, 3 and 4 infected adults. Its chemical name is N,N’-[[1,1′-biphenyl]-4,4′-diylbis[1H-imidazole-5,2-diyl-(2S)-2,1-pyrrolidine Diyl [(1S)-1-(1-methylethyl)-2-oxo-2,1-ethanediyl]]]biscarbamate C,C’-dimethyl ester, literature report 4, The synthesis of 4′-bis(2-bromoacetyl)biphenyl uses biphenyl and bromoacetyl bromide as substrates, CS as the reaction solvent, and Friedel-Crafts acylation reaction under the action of AlCl to obtain the product 4,4′ -Bis(2-bromoacetyl)biphenyl.
CS is used in this synthesis method, which is highly toxic and volatile; the generated HBr needs to be evaporated during the reaction; post-processing requires recrystallization in toluene three times to obtain the product; and the reaction yield is only 14%. The entire reaction process involves highly toxic reagents, cumbersome operations, complex purification processes, extremely low yields, and no possibility of industrialization. Therefore, there is an urgent need in this field to develop a preparation method for 4,4′-bis(2-bromoacetyl)biphenyl with low raw material price, low reagent toxicity and high product yield.
Preparation[1]
The preparation method of 4,4’-bis(2-bromoacetyl)biphenyl includes the following steps:
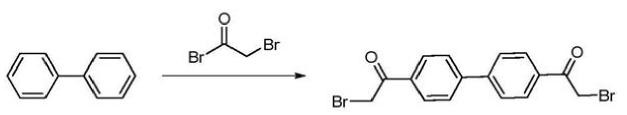
(1) At the reaction temperature of -10℃~30℃, add the catalyst and chlorinated alkane solvent into the reaction bottle and mix. After the system cools down to -10~30℃, add bromoacetyl bromide dropwise into the system, and the dripping is completed. , keep warm and stir for 10 to 30 minutes. A chlorinated alkane solution containing biphenyl is added dropwise to cause a Friedel-Crafts acylation reaction between bromoacetyl bromide and biphenyl to obtain a first mixed system. The chlorinated alkane solvent includes (but is not limited to): methylene chloride, 1,2-dichloroethane, chloroform and/or carbon tetrachloride; the catalyst includes (but is not limited to): trichloride Aluminum, zinc chloride and/or ferric chloride.
(2) Raise the temperature to room temperature and stir continuously, add antisolvent to the first mixed system, and continue the reaction at a reaction temperature of 20°C to 60°C for 0.5 to 8 hours to obtain 4,4′-bis(2- The second mixed system of bromoacetyl)biphenyl; the antisolvent is selected from C3 to C10 alkanes, ethers, and esters; the C3 to C10 alkanes are selected from: n-hexane, n-heptane, cyclohexane, methyl cyclohexane, pentane, octane, nonane, and decane; ethers are selected from: petroleum ether, methyl tert-butyl ether, diethyl ether, ethylene glycol dimethyl ether, tetrahydrofuran, 2-methyltetrahydrofuran, isopropyl Propyl ether; esters are selected from: ethyl acetate, butyl acetate, isopropyl acetate; preferably n-hexane, cyclohexane, n-heptane, petroleum ether; more preferably n-heptane, cyclohexane .
(3) Control the temperature of the second mixed system containing 4,4′-bis(2-bromoacetyl)biphenyl at -5~25°C, stir for 2~5 hours, and filter with suction to obtain 4,4′ -Crude bis(2-bromoacetyl)biphenyl; beat the obtained crude product in water and dichloromethane solution at room temperature respectively, filter and dry to obtain fine product 4,4′-bis(2-bromoacetyl) biphenyl.
Main reference materials
[1] CN201710267562.6 Preparation method of 4,4’-bis(2-bromoacetyl)biphenyl

 微信扫一扫打赏
微信扫一扫打赏

