Background and overview[1]
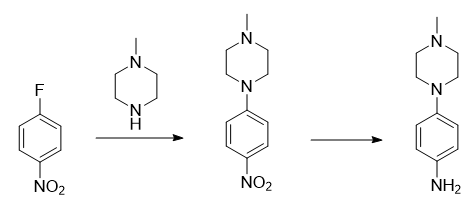
4-(4-methylpiperazine)aniline can be used as a pharmaceutical intermediate. It can be prepared by reducing p-fluoronitrobenzene with N-methylpiperazine after nucleophilic substitution. 4-(4-methylpiperazine)aniline can be used to prepare 2-N-aryl-4-N-aryl-5-fluoropyrimidine compounds. This compound has a high FGFR4 inhibitory effect and can be used as an effective potential anticancer drug. Fibroblast growth factor receptor (FGFR) is a type of transmembrane receptor tyrosine kinase. Currently, the known FGFRs mainly include 4 types, namely FGFR1, FGFR2, FGFR3 and FGFR4. FGFR family members play an important role in controlling cell proliferation and Plays an important role in differentiation signaling pathways.
Preparation[1]
1) Synthesis of 4-(4-methylpiperazine)nitrobenzene
Dissolve 1.0g (7.1mmol) p-fluoronitrobenzene and 1.42g (14.17mmol) N-methylpiperazine in 10mL dry dimethyl sulfoxide (DMSO), and then add to the mixed solution 1.96g (14.17mmol) potassium carbonate. After the reaction was stirred at room temperature for 5 hours, ice water was added to the reaction solution. After precipitation, the mixture was filtered and the filter cake was dried to obtain 1.18g of yellow product with a yield of 75.25%.
2) Synthesis of 4-(4-methylpiperazine)aniline
Dissolve 1.0g 4-(4-methylpiperazine)nitrobenzene in 10mL methanol, slowly add a catalytic amount of 10% Pd/C (mass fraction) while stirring, and pass in hydrogen gas, and react at room temperature for 5 hours. The reaction solution was suction filtered and the filter cake was washed with methanol. The filtrate was spin-dried under reduced pressure to remove methanol, and the residue was separated by silica gel column chromatography to obtain 0.72g of off-white solid 4-(4-methylpiperazine)aniline, with a yield of 83.7%.
References
[1]CN201910895654.8 A 2-N-aryl-4-N-aryl-5-fluoropyrimidine compound and its preparation method and application

 微信扫一扫打赏
微信扫一扫打赏

