Overview[1]
The N-formylation reaction of amines is an important type of reaction in synthetic chemistry, because this conversion provides a direct route to the synthesis of formamide compounds with diverse structures. Formyl groups are widely found in natural products and drug molecules. For example, leucovorin, formoterol and orlistat all have formamide structural units. In addition, N-formyl derivatives such as methylformanilide are very useful reagents in the Vilsmeier formylation reaction and are often used as precursors for the synthesis of isocyanides, formamidines, arylamides, isocyanates, nitriles and other compounds.
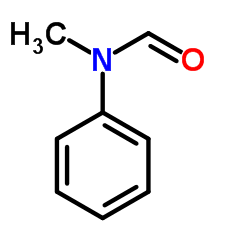
Currently, many formylation reagents are used to achieve the N-formylation reaction of amines, such as formaldehyde, paraformaldehyde, methanol, ammonium formate, etc. However, among the above-mentioned N-formylation reagents, when formaldehyde and paraformaldehyde react, they will generate polluting gases, pollute the environment, and affect the experimenter’s own health; ammonium formate has shortcomings such as easy water absorption and deliquescence; when using methanol for N-formylation, During the formylation reaction, the reaction time was too long, reaching 24 hours; various formylation reagents have certain limitations.
Preparation[1]
Methylformanilide is prepared as follows:
Method 1: (1) Weigh 22.6g of ZrOCl2.8H2O and dissolve it in 77.4g of a mixed solution of deionized water and ethanol (mass ratio is 1:1) to prepare zirconium oxychloride with a mass concentration of 12.5 % solution; weigh 14.7g trimesic acid (H3BTC) and dissolve it in a mixed solution of 85.3g ethanol and N, N dimethylformamide (DMF) (preferably the mass ratio is 1:1) to prepare benzene. A solution with a mass concentration of triformic acid of 14.7%. While stirring, add the zirconium oxychloride solution and trimesic acid solution dropwise into the reactor, where the molar ratio of the metal ions of zirconium oxychloride to trimesic acid is 1:1. Stir thoroughly, then ultrasonic for 50 minutes at 180W ultrasonic power, then crystallize at room temperature for 48 hours, centrifuge, wash with deionized water and ethanol, and vacuum dry at 150°C for 12 hours to obtain powder MOF-808 ( Zr) material, recorded as MOF-808(Zr)2.
(2) Weigh 19.1g of MOF-808(Zr)2 and dissolve it in 80.9g of ethanol to prepare a dispersion of MOF-808(Zr) with a mass concentration of 19.1%, which is recorded as liquid A2. Weigh 9.5g of RuCl3 and dissolve it in 90.5g of ethanol-water mixture (mass ratio is 1:1) to prepare a metal precursor solution with a Ru mass content of 4.65wt%, recorded as liquid B2. Under stirring, add 20g of B2 liquid dropwise into the A2 liquid, stir thoroughly, and then ultrasonic for 55 minutes under 80W ultrasonic power. During this period, the metal ions can be gradually and gently reduced by ethanol, and the assembly of nanometallic materials in MOF-808 (Zr) is completed. the process of. After filtration, it was washed with deionized water and ethanol, and vacuum dried at 150°C for 12 hours to obtain the 4.65wt% Ru/MOF-808 (Zr) solid catalyst.
(3) In a 100mL kettle-type high-pressure reactor, add 5mL of N-methylaniline, then add 0.0022g of the prepared 4.65wt% Ru/MOF-808 (Zr) solid catalyst, and then fill in 3.2MPa CO2 and H2 (volume ratio is 1:1) mixed gas, and then reacted at 200°C for 8 hours. After the reaction was completed, it was determined through chromatographic analysis that the conversion rate of aniline was 68.25% and the selectivity of methylformanilide was 98.31%
Method 2: Combine 21.4 mg (0.2mmol) N-methylaniline, 81.2 mg ethyl bromodifluoroacetate (0.4mmol), 2.5 mg (0.02mmol) cuprous acetate, 19.1 mg (0.04mmol) -phos, 32.7 mg (0.2 mmol) cesium carbonate, added to 2 mL DMF solvent. React at 110°C for 12 hours. After the reaction is completed, cool and filter. The filtrate is evaporated to remove the solvent. The residue is subjected to silica gel column chromatography and washed with a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 8:1. Press The effluents were collected from the actual gradient and detected by TLC. The effluents containing the product were combined, the solvent was distilled off with a rotary evaporator, and dried under vacuum to obtain 22.2 mg of yellow liquid methylformanilide with a yield of 82%.
Main reference materials
[1] CN201910616373.4 A MOF-808 (Zr) assembled nanometal catalyst with CUS, preparation and application
[2] CN201810720447.4 An N-arylformamide prepared by using ethyl bromodifluoroacetate as the formylation reagent

 微信扫一扫打赏
微信扫一扫打赏

