Background and overview[1]
4-Iodo-2,6-dimethylaniline is an organic intermediate that can be prepared from iodination of 2,6-dimethylaniline. The amino group of 4-iodo-2,6-dimethylaniline can be diazotized to prepare dimethyl-p-bromoiodobenzene. Dimethyl p-bromoiodobenzene is an aromatic functional monomer with two different halogen groups. Taking advantage of the difference in reactivity of bromine and iodine, it can react with different groups respectively to synthesize compounds substituted by various functional groups. It is widely used in organic synthesis, metal-organic chemistry, linear aromatic conductive polymers, new macrocyclic compounds, etc. It is an important aromatic organic synthesis intermediate.
Preparation[1]
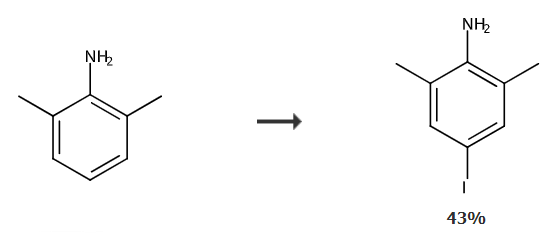
Add fully ground iodine 12.7g (0.05mol), 40mL ethyl acetate and 50mL acetic acid into a 250mL three-neck flask, add SPC 7.5g (0.054mol) in batches while stirring, and complete the addition within 20 minutes. Then add 12.4 mL (0.1 mol) of 2,6-dimethylaniline, stir at 20-25°C for 0.5h, then raise the temperature to 45-50°C and reflux for 1 hour. After cooling, pour the mixture into 200mL w(Na2SO3)=5% aqueous solution. After stirring thoroughly, the organic phase is evaporated under reduced pressure to remove the solvent to obtain black purple color. The solid crude product was recrystallized from n-hexane to obtain 10.5g of white needle-like crystals 4-iodo-2,6-dimethylaniline, yield 42.5%
Apply[1]
4-iodo-2,6-dimethylaniline is used to prepare 3,5-dimethyl-4-bromoiodobenzene. The method is as follows: add 4.9g of 4-iodo-2,6-dimethylaniline (0.02mol), 100mL water, w(H2SO4)=98% sulfuric acid 2.24mL (0.04mol) was added to the 250mL four-neck flask, stir and cool to -5~0℃, at this temperature, slowly add a solution of 1.75g NaNO2 (0.025mol) dissolved in 30mL water (use starch-KI test paper to detect the reaction end point). After the dripping is completed, pour the diazonium salt solution into 2.9g CuBr (0.02mol) and dissolve it in 2.7mL w (HBr) = 40% hydrobromic acid (0.02mol). Stir and heat to 45~50°C, and react at this temperature. 0.5h. After cooling, the mixture was refluxed and extracted with 50 mL of n-hexane. The organic phase was washed with saturated NaCl aqueous solution, washed with water, and dried with anhydrous MgSO4. After the solvent was evaporated under reduced pressure, a reddish-brown solid crude product was obtained. Recrystallization from water and ethanol gave 1.9 g of off-white solid 3,5-dimethyl-4-bromoiodobenzene, with a yield of 31.5%.
Main reference materials
[1] Liu Rui, Li Yuhao, Xiao Benliang, Liu Shan, Zhu Hongjun. Synthesis of 3,5-dimethyl-4-bromoiodobenzene and 2,6-dimethyl-4-bromoiodobenzene[J ]. Fine Chemicals, 2008(03):304-307.

 微信扫一扫打赏
微信扫一扫打赏

