【Name】Dichloro[1,1′-bis(diphenylphosphino)ferrocene]palladium(II),
Dichloro[1,1′-bis(diphenylphosphine)ferrocene]palladium
[Molecular formula] C34H28Cl2FeP2Pd
[Molecular weight] 731.72
[CA registration number][72287-26-4]
[Abbreviation and alias] PdCl2(dppf)
[Physical properties] Reddish brown solid, mp 265~268oC. Soluble in ether, THF and benzene.
[Preparation and commercial products] PdCl2 (dppf) has been commercialized and is sold by major reagent companies.
【Note】This reagent is irritating and should be stored in an inert atmosphere.
————————————————– ———
PdCl2 (dppf) is mainly used as a catalyst to catalyze cross-coupling reactions.
Similar to other Pd(II) and Ni(II) complexes, PdCl2(dppf) can effectively catalyze the cross-coupling reaction between halogenated alkenes, halogenated arenes or triflate aromatics and Grignard reagents. Achieve the formation of carbon-carbon bonds. Vinyl bromide and s-BuMgCl can react completely under the catalysis of PdCl2 (dppf) to obtain the coupling product (Formula 1)[1], but using other Pd catalysts will lead to isomeric products and reduction by-products.

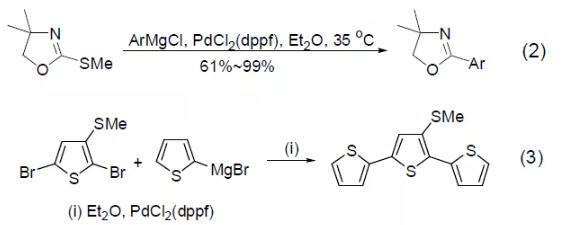
Although ordinary Pd catalysts do not have the ability to activate organic sulfur compounds, PdCl2 (dppf) can realize the cross-coupling reaction between methyl sulfide and aryl Grignard reagents (Formula 2)[2]. Bromothiophene and thiophene Grignard reagent can obtain photosensitive trithiophene compounds under the catalysis of PdCl2 (dppf), and the methyl sulfide substituent in the substrate will not react (Formula 3) [3]. 【Kumada coupling reaction】
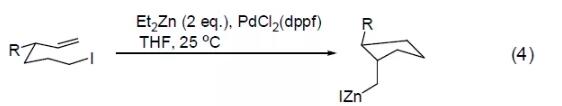
PdCl2(dppf) was also found to catalyze the iodine-zinc exchange reaction to obtain the intramolecular carbon zincation reaction of alkenes (Equation 4)[4].
PdCl2 (dppf) reagent can also catalyze Stille reaction and Suzuki reaction, such as the cross-coupling reaction between vinyl tin and aryl trifluoromethanesulfonic acid to obtain an intramolecular cyclization product (Formula 5) [5] , or a cross-coupling reaction between aryltin and aryltrifluoromethanesulfonic acid in a CO atmosphere to obtain an aryl ketone compound (Formula 6) [6]. [Examples of preparation of carboxylic acids and their derivatives by carbonyl insertion reaction]

PdCl2 (dppf) can also be used in the hydroesterification reaction of trimethylsilyl alkyne to obtain conjugated vinyl silicon compounds (Formula 7) [7].
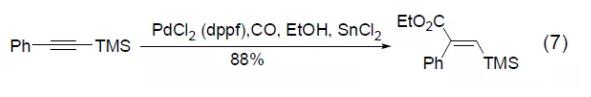
References
1. (a) Hayashi, T.; Konishi, M.; Kumada, M. Tetrahedron Lett., 1979, 1871. (b) Hayashi, T.; Konishi, M.; Kobori, Y.; Kumada, M.; Higuchi, T.; Hirotsu, K. J. Am. Chem. Soc., 1984, 106,158.
2. Pridgen, L. N.; Killmer, L. B. J. Org. Chem., 1981, 46, 5402.
3. (a) Rossi, R.; Carpita, A.; Ciofalo, M.; Lippolis, V. Tetrahedron, 1991, 47, 8443. (b) Carpita, A.; Rossi, R.;Veracini, C. A. Tetrahedron, 1985, 41, 1919.
4. Stadtmuller, H.; Lentz, R.; Tucker, C. E.; Studeman, T.;Dorner, W.; Knochel, P. J. Am. Chem. Soc., 1993, 115, 7027.
5. Luo, F.-T.; Wang, R.-T. Tetrahedron Lett., 1991, 32, 7703.
6. Echavarren, A. M.; Stille, J. K. J. Am. Chem. Soc., 1988, 110,1557.
7. Takauchi, R.; Sugiura, M.; Ishii, N.; Sato, N. Chem. Commun.,1992, 1358.
Reprinted from: “Modern Organic Synthesis Reagents—Properties, Preparation and Reactions”, edited by Hu Yuefei and others

 微信扫一扫打赏
微信扫一扫打赏

