Background and overview[1][2]
6-Phenylhexanoic acid is an acidic organic compound and is used as a pharmaceutical intermediate. It can be prepared from 2-benzoylcyclopentanone in two steps.
Preparation method[1-2]
Report 1,
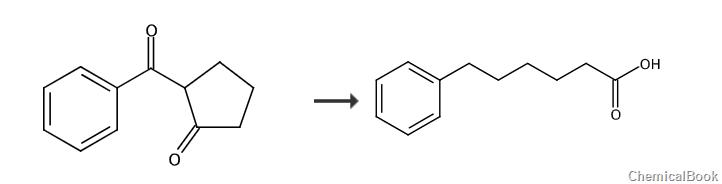
5.65 g of 2-benzoylcyclopentanone (30 mmol, 1.0 equiv) was suspended in a solution consisting of 5.04 g of potassium hydroxide (90 mmol, 3.0 equiv) and 260 ml of water. Heat the reaction system to a reflux state and maintain reflux until the reaction system becomes a homogeneous phase. Cool to room temperature, and adjust the pH value of the system to 4 with hydrochloric acid to precipitate compound 6-oxo-6-phenylhexanoic acid (4.94 g, crude yield: 80%). Recrystallization from isopropanol afforded pure 6-oxo-6-phenylhexanoic acid (3.71 g, yield: 60%). 1H NMR (300MHz, CDCl3) δ7.57-7.37 (m, 5H), 2.92 (t, J=7.5Hz, 2H), 2.33 (t, J=7.5Hz, 2H), 1.74-1.56 (m, 2H), 1.40-1.32 (m, 2H).
4.18 g of 6-oxo-6-phenylhexanoic acid (20.25 mmol, 1.0 equiv) was dissolved in 100 ml of absolute ethanol, after which 1.40 g of 10% palladium on carbon was added. The reaction system was placed under a hydrogen atmosphere and heated to 65°C. After reacting for 18 hours, filter and concentrate the filtrate to obtain 3.89 g of phenyhexanoic acid (yield: 100%). 1H NMR (300MHz, CDCl3) δ7.29-7.25 (m, 2H), 7.19-7.16 (m, 3H), 2.60 (t, J=7.5 Hz, 2H), 2.35 (t, J=7.5Hz, 2H), 1.67-1.55 (m, 4H), 1.41-1.37 (m, 2H).
Report 2,
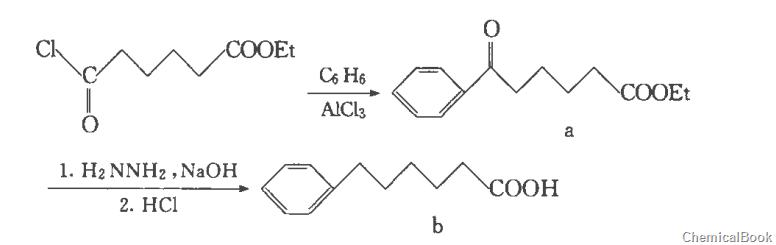
The first step, synthesis of ethyl 5-benzoylvalerate
Add 75 ml of benzene and 13.3 g of anhydrous aluminum trichloride (0.1 mol) into a 250 ml three-necked flask equipped with a condensation reflux tube, a constant pressure dropping funnel, a thermometer, and a calcium chloride drying tube, and stir in an ice-salt bath. Mix, slowly add 18g (0.1mol) of monoethyl adipate acid chloride dropwise at 0~5°C, and complete the dropwise addition in 2 hours. Reflux the reaction for 6 hours and cool to room temperature. Pour the reaction solution into a mixture of 200 ml ice water and 20 ml concentrated hydrochloric acid, stir for 20 minutes, extract the organic phase with ethyl acetate, wash with saturated brine, dry over anhydrous magnesium sulfate, filter, and evaporate the filtrate to obtain 5-benzyl as a yellow oil. Ethyl acylvalerate.
The second step, synthesis of phenyhexanoic acid
Add 100ml diethylene glycol (1.05mol), 23.4g (0.1mol) ethyl 5-benzoylvalerate, and 30ml 80% hydrazine hydrate into a 500ml four-neck bottle equipped with a condensation reflux tube. After mixing, heating and refluxing for 2 hours, lower the temperature and install a water separation device. Steam out and collect the water and unreacted hydrazine hydrate below 180°C. Stop heating. After the reactants have cooled, add 8g (0.2mol) of 80% hydrogen into the system. Sodium oxide aqueous solution, heat the reaction below 210°C, and collect the water generated by the reaction at the same time. When the reaction rises to 210°C, remove the water separation device, reflux for 3 hours and then cool to room temperature. Use cold dilute hydrochloric acid to acidify the mixture until no new water is generated. Until precipitation, the product phenyhexanoic acid is obtained by suction filtration.
Main reference materials
[1] [China invention, China invention authorization] CN201010034402.5 6/7-(hetero)aryl-N-hydroxyhexan/heptyl amide compound and its preparation method
[2] Dong Li, Zhang Shuwen, Lu Hongkai, Shi Minyu. Research on the synthesis of 1-bromo-6-phenylhexane [J]. Fine and Specialty Chemicals, 2010, 18(07): 29-31 .

 微信扫一扫打赏
微信扫一扫打赏

