Background and overview[1][2]
Also called 2,4,6-triphenol-1,3,5-s-triazine. Belongs to a class of small molecules, branched and polymer materials containing special triazine units, which are used in organic electroluminescence, organic field effect tubes, organic solar cells, nonlinear optics, biosensing, organic light storage and Organic lasers and other fields have a wide range of applications.
Organic optoelectronic functional materials such as liquid crystals, nonlinear optics and electroluminescence derived from 1,3,5-s-triazine have star-shaped, hyperbranched and dendritic three-dimensional structures. Among this type of macromolecules, symmetrically substituted 2,4,6-triphenoxy-1,3,5-s-triazine is a basic structural unit containing a variety of functional groups. Phenoxy-1,3,5-s-triazine derivatives are used as functional modules to construct macromolecular organic photoelectric functional materials.
Preparation[1-2]
Report 1,
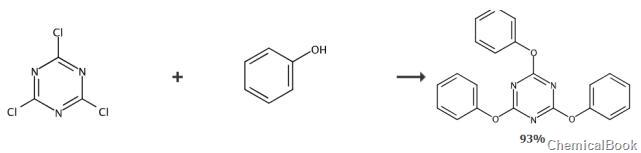
Add 0.5g trichlorotriazine, 0.84g phenol and 0.37g sodium hydroxide solid into a two-necked bottle, vacuum and fill with nitrogen, then add 30ml of acetone/water (1:1) mixture, react at 80°C for 24h , it was filtered and recrystallized directly after the reaction. The yield is 93%
Report 2,
When KOH (36 mmol), phenol (18 mmol) and cyanuric chloride (5 mmol) are mixed and ground, HCl gas is immediately generated, accompanied by an exothermic reaction. After grinding for 5 minutes, the mixture changed from light yellow paste to solid powder. Pour the crude product into water, stir, filter, wash with water until neutral, dry, and recrystallize in a mixed solvent of dichloromethane and ethanol to obtain 2 , 4,6-triphenoxy-1,3,5-s-triazine white needle-like crystals.
Main reference materials
[1] [Invented in China] CN200910232492.6 Triazine photoelectric functional materials and preparation and application methods
[2] Luo Chunhua, Yu Meina, Zhao Ling, Cheng Ge. Synthesis of 2,4,6-triphenoxy-1,3,5-s-triazine derivatives by solid-state reaction [J]. Applied Chemistry, 2005(07):811-812.

 微信扫一扫打赏
微信扫一扫打赏

