Background and overview[1]
With the development of metal-organic chemistry, transition metal-catalyzed coupling reactions have become one of the most effective means of constructing C-C, C-N and C-O bonds. As a key factor in the coupling reaction, ligands can not only stabilize the metal center, but also effectively activate the metal center. Therefore, they have received great attention from researchers.
xantphos (4,5-bisdiphenylphosphine-9,9-dimethylxanthene) compound is used in palladium-catalyzed Suzuki and Negishi due to its special electron-rich property and large steric hindrance ability. It has high reactivity in coupling reactions such as Stille and Heck. So far, the synthesis methods of 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (xantphos) compounds reported in the literature mainly use 9,9-dimethylxanthene via n-butyl After lithiation with lithium or isopropyllithium, it reacts with tert-butylphosphine chloride and is quenched with methanol to obtain 4,5-bisdiphenylphosphine-9,9-dimethylxanthene ( xantphos) compounds. The main disadvantage of this method is that n-butyllithium or isopropyllithium is required in the reaction. The anhydrous and oxygen-free operation during the reaction requires higher equipment and is more dangerous to operate. In view of the superior catalytic properties and broad market application prospects of this type of compounds, it is necessary to explore more efficient and practical synthesis methods of 4,5-bisdiphenylphosphine-9,9-dimethylxantphos compounds. .
Preparation method[1]
Method 1: 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (xantphos) is prepared as follows:
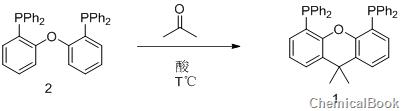
Under argon protection, add 538g of bis(2-diphenylphosphenyl) ether (compound 2), 700mL of toluene solvent, and 8.8mL of trifluoromethanesulfonic acid (bis(2-diphenyl)) into the reaction kettle. Phosphophenyl) ether (compound 2) and trifluoromethanesulfonic acid (the molar ratio is 1:0.1), 150 mL of acetone, heat the reaction system to 150oC, react for 16 hours, then reduce to room temperature, extract, dry, and recrystallize 520 g of the target product 4,5-bisdiphenylphosphine-9,9-dimethylxantphos (xantphos) (compound 1) was obtained (yield 90%). 1HNMR (CDC13): 7.40 (dd, 2H, J=7.8, 1.0Hz), 7.15-7.26 (20H), 6.96 (t, 2H, J=7.7Hz), 6.54 (dd, 2H, J=7.4, 1.4Hz ), 1.65(s, 6H, CH3).31P{1H}NMR(CDC13): -17.5.13C{1H}NMR(CDC13): 137.2(t, J=5.3Hz), 133.7(t, J=10.1Hz ), 131.9, 129.7, 128.0, 126.1, 125.7 (t, J=9.8Hz), 123.1, 67.8, 31.6.
Method 2: 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (xantphos) is prepared according to the above reaction formula, specifically:
(1) Dilute 9,9-dimethylxanthene (0.02mol) and tetramethylethylenediamine (TMEDA) (42mmol) in hexane (50mL) at -78°C. Add n-BuLi (42mmol) hexane solution dropwise (complete within about 5 minutes), react for 1 hour, then rise to room temperature (about 25°C), and continue stirring for 16 hours;
(2) Then add 15 mL of n-hexane solution of diphenylphosphine chloride (42 mmol) dropwise into the reaction system of step (1) under an ice-water bath (completed within about 5 minutes), and then continue to add at room temperature ( The reaction was stirred for 16 hours at about 25°C); the solvent was removed by rotary evaporation to obtain a light yellow viscous oil, which was washed with acetone and dried under vacuum to obtain 9.7g of white powder, namely compound xantphos: the yield was 84%, 1HNMR (400MHz, C6D6) δ: 7.45-7.33 (m, 8H, o-PPh2), 7.08 (dd, 2H, J=7.7, 1.4Hz, PCCHCH), 7.05-6.95 (m, 12H, m-PPh2, p-PPh2), 6.84 (dd, J=7.5, 1.5Hz, 2H, CHCHCC), 6.76 (t, J=7.6Hz, 2H, CHCHCH), 1.37 (s, 6H, CH3)ppm.
Main reference materials
[1] CN201910328132.X A synthesis method of 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (xantphos)

 微信扫一扫打赏
微信扫一扫打赏

