Background and overview[1]
Me-hydroxyphenylacetic acid can be used as a pharmaceutical synthesis intermediate. Meta-hydroxyphenylacetic acid can be prepared by the hydrolysis of aryl halides. Copper-catalyzed hydrolysis of aryl halides without the use of phase transfer catalysts has very important application prospects. Appropriate ligands are the key to the occurrence of this type of reaction. Transition metal-catalyzed hydrolysis of aryl halides is an efficient method for the preparation of phenols. Metal palladium has high efficiency in catalyzing the hydrolysis reaction of halogenated aromatic hydrocarbons, but palladium catalysts are expensive, and usually large sterically hindered and structurally complex phosphine ligands or azacarbene ligands need to be added to the reaction system to promote reaction, which limits its application to a certain extent. This field still lacks a green and suitable for industrial application, a copper-catalyzed catalytic system that can efficiently carry out the aryl halide hydrolysis reaction.
Preparation[2]
Preparation of m-hydroxyphenylacetic acid: In a 100mL hydrothermal synthesis reaction kettle, add sodium hydroxide (3mmol) and water (5mL), stir and dissolve, add m-iodophenylacetic acid (0.5mmol), cuprous oxide ( 0.05mmol), ascorbyl alcohol (0.05mmol), the reaction was stirred evenly at 100°C for 6 hours. After cooling, adjust the pH to 2 with dilute hydrochloric acid, and then extract with ethyl acetate. The extract was concentrated and then subjected to column chromatography to obtain Hydroxyphenylacetic acid, 54.0 mg, yield 71%. m/z:153.1[M+H]+, 150.9[M-H]-, m.p.129-131℃.
Apply[1]
M-hydroxyphenylacetic acid can be used to prepare 3-ethoxy-4-ethoxycarbonylphenylacetic acid:
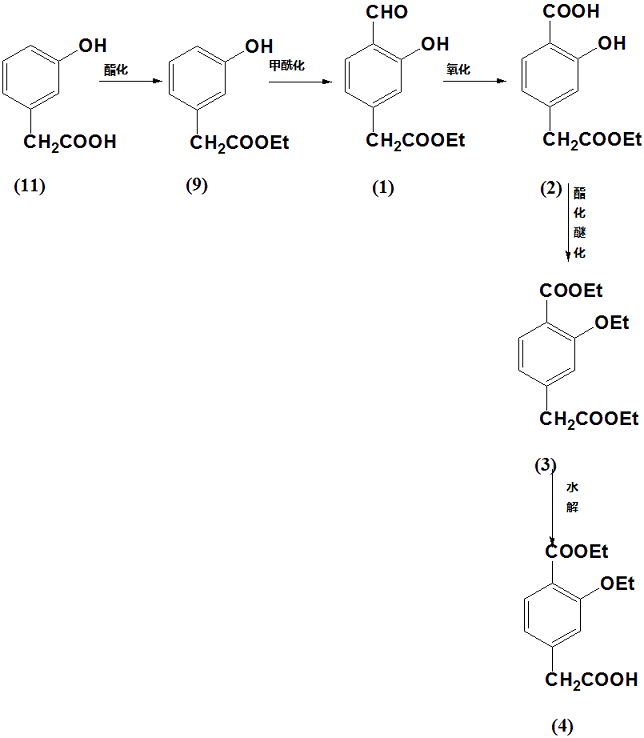
Using compound 11 m-hydroxyphenylacetic acid as raw material, ethyl m-hydroxyphenylacetate is first synthesized through esterification reaction, which is compound 9, and then 4-formyl-3-hydroxyphenylacetic acid ethyl ester is synthesized through formylation reaction. 1. After oxidation reaction, 4-acetyl ethoxy-2-hydroxybenzoic acid is synthesized, which is compound 2, and then esterification and etherification reaction is performed to synthesize ethyl 3-ethoxy-4-ethoxycarbonylphenylacetate, which is compound 3. Finally, compound 4 was obtained through hydrolysis reaction.
Main reference materials
[1] CN201910303215.3 Application of quinoa alcohol in copper-catalyzed aryl halide hydrolysis reaction
[2] CN201410091929.Synthesis method of X3-ethoxy-4-ethoxycarbonylphenylacetic acid

 微信扫一扫打赏
微信扫一扫打赏

