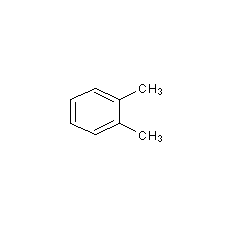
Structural formula
| Business number | 0294 |
|---|---|
| Molecular formula | C8H10 |
| Molecular weight | 106 |
| label |
1,2-Dimethylbenzene, 1,2-Dimethylbenzene, aromatic compounds |
Numbering system
CAS number:95-47-6
MDL number:MFCD00008519
EINECS number:202-422-2
RTECS number:ZE2450000
BRN number:1815558
PubChem number:24890181
Physical property data
1. Properties: Colorless transparent liquid with an odor similar to toluene. [1]
2. Melting point (℃): -25[2]
3. Boiling point (℃): 144.4[3]
4. Relative density (water = 1): 0.88[4]
5. Relative vapor Density (air=1): 3.66[5]
6. Saturated vapor pressure (kPa): 1.33 (32℃)[6]
7. Heat of combustion (kJ/mol): -4845.3[7]
8. Critical temperature (℃): 359[8]
9. Critical pressure (MPa): 3.7[9]
10. Octanol/water partition coefficient: 3.12 [10]
11. Flash point (℃): 16 (CC) [11]
12. Ignition temperature (℃) ): 463[12]
13. Explosion upper limit (%): 7[13]
14. Explosion lower limit (%): 0.9[14]
15. Solubility: Insoluble in water, miscible in most organic solvents such as ethanol, ether, and chloroform. [15]
16. Viscosity (mPa·s, 25ºC): 0.754
17. Flash point (ºC): 495.5
18. Heat of evaporation (KJ/mol, 101.3kPa): 36.84
19. Heat of evaporation (KJ/mol, 0.889kPa): 43.463
20. Heat of fusion (KJ/ mol, 101.3kPa): 13.607
21. Heat of formation (KJ/mol, 25ºC, gas): 19.008
22. Heat of formation (KJ/mol, 25ºC, liquid): -24.455
23. Heat of combustion (KJ/mol, 25ºC, gas): 4599.36
24. Heat of combustion (KJ/mol, 25ºC, liquid): 4555.90
25. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 1.26
26. Thermal conductivity (×10-4 W/(m ·K),16<t<91ºC): (0.1320~1.6979)×10-4t
27. Relative density (20℃, 4℃): 0.8801
28. Refractive index at room temperature (n20): 1.5055
29. Critical density (g·cm-3): 0.287
30. Critical volume (cm3·mol-1): 370
31. Critical compression factor: 0.263
32. Eccentricity factor: 0.303
33. Lennard-Jones parameter (A): 15.06
34. Lennard-Jones parameter (K): 169.9
p>
35. Solubility parameter (J·cm-3)0.5: 18.453
36. van der Waals area (cm2·mol-1): 8.840×109
37. van der Waals volume (cm3 ·mol-1): 70.660
38. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): – 4596.29
39. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): 19.08
40. Gas phase standard entropy (J·mol-1·K-1): 353.94
41. Gas phase standard free energy of formation (kJ·mol-1) : 122.1
42. Gas phase standard hot melt (J·mol-1·K-1): 132.31
43. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -4552.86
44. Liquid phase standard claimed heat (enthalpy) )( kJ·mol-1): -24.35
45. Liquid phase standard entropy (J·mol-1·K– 1): 246.61
46. Liquid phase standard formation free energy (kJ·mol-1): 110.46
47. Liquid phase Standard hot melt (J·mol-1·K-1): 187.65
Toxicological data
1. Acute toxicity[16]
LD50: 4300mg/kg (rat oral); 1364mg/kg (mouse intravenous )
LC50: xylene, 5000ppm (rat inhalation, 4h)
2. Irritation [17]
Rabbit transdermal: xylene, 500mg (24h), moderate irritation.
Rabbit eye: xylene, 87mg, mild irritation: 5mg (24h), severe irritation.
Human eye: xylene, 200ppm, irritating.
3. Subacute and chronic toxicity [18] Rat and rabbit inhalation concentration 3000mg/m3, daily 8 hours, 6 days a week for a total of 130 days, mild leukopenia occurred, with no change in red blood cells and platelets.
4. Teratogenicity[19] The lowest toxic dose of inhalation (TCLo) in rats 7~14 days after pregnancy is 3000mg/m3 (24h), causing developmental malformations of the musculoskeletal system.
5. Carcinogenicity [20] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals.
6. Others[21]
The lowest toxic concentration for inhalation in rats (TCLo) : 1500mg/m3 (24h) (administered on 7th to 14th day of pregnancy), has embryotoxicity.
Xylene, human oral LCLo: 50mg/kg; human inhalation TCLo: 200ppm; LCLo: 10000ppm (human inhalation, 6h)
Ecological data
1. Ecotoxicity[22]
LC50: 13mg/L (24h), 16.9ppm/96h (goldfish); 42mg/ L (96h) (fathead minnow, static); 13mg/L (96h) (rainbow trout); 100~1000mg/L (24h) (water flea)
EC50: 97mg/L (5min) (Photoluminous bacteria, Microtox toxicity test)
2. Biodegradability[23]
Aerobic biodegradation (h ): 168~672
Anaerobic biodegradation (h): 4320~8640
3. Non-biodegradability [24]
Maximum light absorption wavelength range of photolysis (nm): 262~269.5
Photooxidation half-life in water (h): 3.90×105 ~2.70×108
Half-life of photooxidation in air (h): 4.4~44
4. Other harmful effects[ 25] Its environmental pollution behavior is mainly reflected in drinking water and the atmosphere. The residue and accumulation are not serious. It can be biodegraded and chemically degraded in the environment, but this process is faster than the volatilization process. At a much lower rate, xylene volatilized into the atmosphere may also be photolyzed.
Molecular structure data
1. Molar refractive index: 35.90
2. Molar volume (cm3/mol): 121.9
3. Isotonic specific volume (90.2K ): 282.5
4. Surface tension (dyne/cm): 28.7
5. Polarizability (10-24cm3): 14.23
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 56.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Oxidation of dilute nitric acid produces o-toluic acid. Oxidation of potassium permanganate yields phthalic acid and o-toluic acid. Using vanadium pentoxide as a catalyst, vapor phase oxidation occurs at 480°C to generate phthalic anhydride. Xylene is boiled under photocatalysis and chlorine gas is introduced to generate chlorinated xylene (CH3C6H4CH2Cl); when chlorine is continued to be introduced, the hydrogens on both sides of the chain can be replaced by chlorine in turn.
2. Stability[26] Stable
3. Incompatible substances[27] Strong oxidants, halogens
4. Polymerization hazard[28] No polymerization
Storage method
Storage Precautions[29] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Super distillation is used industrially to separate o-xylene from mixed xylene. The boiling points of o-xylene and other components in mixed xylene differ by more than 5°C. Distillation requires about 150 plates and a reflux ratio of 5-8, which requires a lot of energy. It is produced by fractionation of the light oil part of fractionated coal tar or catalytic reforming of light oil, or by disproportionation of toluene.
2. Use industrial o-xylene as raw material, first wash with industrial concentrated sulfuric acid until the acid layer is colorless, then wash with 10% sodium hydroxide solution and water in sequence until it is qualified, separate the water layer and use The calcium chloride hydrate is dried and then distilled. After the distillate is clear, the middle fraction is collected, which is the pure product.
Purpose
1. Mainly used as chemical raw materials and solvents. It can be used to produce phthalic anhydride; dyes; pesticides and drugs, such as vitamins, etc. It can also be used as aviation gasoline additive.
2. In addition to being used as a solvent, it is also used as a raw material for the manufacture of phthalic anhydride, phthalonitrile, xylenol and xyluidine, and as an additive for aviation gasoline.
3. Mainly used as solvent and for synthetic paints and coatings. [30]
��It is produced by disproportionation of toluene.
2. Use industrial o-xylene as raw material, first wash with industrial concentrated sulfuric acid until the acid layer is colorless, then wash with 10% sodium hydroxide solution and water in sequence until it is qualified, separate the water layer and use The calcium chloride hydrate is dried and then distilled. After the distillate is clear, the middle fraction is collected, which is the pure product.
Purpose
1. Mainly used as chemical raw materials and solvents. It can be used to produce phthalic anhydride; dyes; pesticides and drugs, such as vitamins, etc. It can also be used as aviation gasoline additive.
2. In addition to being used as a solvent, it is also used as a raw material for the manufacture of phthalic anhydride, phthalonitrile, xylenol and xyluidine, and as an additive for aviation gasoline.
3. Mainly used as solvent and for synthetic paints and coatings. [30]

 微信扫一扫打赏
微信扫一扫打赏

