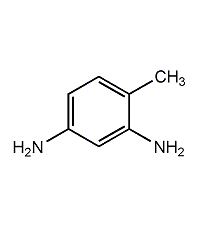
Structural formula
| Business number | 029W |
|---|---|
| Molecular formula | C7H10N2 |
| Molecular weight | 122.17 |
| label |
4-Methyl-1,3-phenylenediamine, 4-methyl-m-phenylenediamine, Toluene-2,4-diamine, m-Toluenediamine, 3-amino-4-methylaniline, 4-methyl-1,3-phenylenediamine, 4-methyl-phenylenediamine, Hardener, intermediates, Aromatic compounds and their derivatives |
Numbering system
CAS number:95-80-7
MDL number:MFCD00007804
EINECS number:202-453-1
RTECS number:XS9625000
BRN number:2205839
PubChem number:24846558
Physical property data
1.Characteristics: colorless needle-shaped or rhombus crystals. [1]
2. Melting point (℃): 97~99[2]
3. Boiling point (℃) :292[3]
4. Saturated vapor pressure (kPa): 0.13 (106.5℃)[4]
5. Critical pressure (MPa): 4.38[5]
6. Octanol/water partition coefficient: 0.337[6]
7. Ignition temperature (℃): 477[7]
8. Solubility: soluble in water, easily soluble in ethanol, ether, and benzene. [8]
Toxicological data
1. Acute toxicity[9] LD50: 590mg/kg (rat oral, 24h); 650mg/kg (rabbit dermal, 24h)
2. Irritation [10]
Rabbit transdermal: 500mg (24h), mild irritation.
Rabbit eye: 100mg (24h), severe irritation.
3. Mutagenicity [11] Microbial mutagenicity: Salmonella typhimurium 100μg/dish. DNA damage: human fibroblasts 100 μmol/L. Unprogrammed DNA synthesis: human liver 100 μmol/L. Micronucleus test: rats take 300mg/kg orally.
4. Carcinogenicity [12] IARC Carcinogenicity Comment: G2B, suspected human carcinogen.
Ecological data
1. Ecotoxicity[13]
LC50: 1420mg/L (96h) (fathead minnow)
EC50: 1290~1440mg/L (96h) (fathead minnow)
2. Biodegradability[14]
Aerobic biodegradation (h): 672~4320
Anaerobic biodegradation (h): 2688~17280
3. Non-biodegradability [15]
Photooxidation half-life in water (h): 31~1740
Photooxidation half-life in air (h): 0.27~2.7
p>
Molecular structure data
1. Molar refractive index: 39.55
2. Molar volume (cm3/mol): 110.2
3. Isotonic specific volume (90.2K ): 296.5
4. Surface tension (dyne/cm): 52.3
5.After that, suck the supernatant liquid into the storage tank, add 1000-1200L of water to the iron mud remaining in the reduction kettle, stir and raise the temperature to 95°C and let it stand for 0.5h, then suck the supernatant washing liquid into the storage tank, repeat the above method Wash three times, and the washing liquid is combined into the reduction mother liquor for suction filtration; after suction filtration, the material liquid is evaporated and concentrated under a vacuum of 80kg, which takes about 6 hours to complete. (4) Distillation under reduced pressure: Suction the concentrated liquid into the distillation kettle, and perform dehydration under stirring at a vacuum degree of 74.7-90.7kpa, an interlayer oil temperature of 180-210°C, and a top temperature of 50-60°C. When the vacuum degree rises to 94.7-97.3kpa, the top temperature is 190-200°C, and the interlayer oil temperature is 280-290°C for distillation. As the material in the distillation kettle decreases, the vacuum gradually rises, and the top temperature gradually drops to 170-180°C. ℃, the oil temperature increases to about 300-320 ℃, at which point the distillation can be considered to have reached the end. Each batch of finished products is about 500kg. Raw material consumption quota: p-nitrotoluene 1600kg/t, sulfuric acid (98%) 1616kg/t, nitric acid (98%) 760kg/t, iron powder (90%) 2700kg/t. 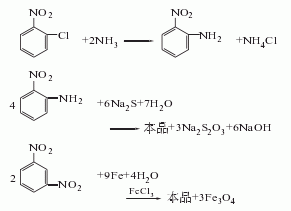
Purpose
1. Used as a curing agent for epoxy resin, the reference dosage is 8 parts by mass. The curing conditions are 100℃/2h+150℃/2h, and the thermal change temperature of the cured product is 150-160℃. It is also used as an intermediate for organic synthesis and dyes, such as the manufacture of fur black DB, sulfurized yellow brown 5G, sulfurized red brown BIR and other dyes. Reaction with phosgene can produce 2,4-toluene diisocyanate.
2. It is one of the raw materials for organic synthesis and can be used to prepare toluene diisocyanate. It is also used as a dye intermediate and for hair dyeing. [22]

 微信扫一扫打赏
微信扫一扫打赏

