Background and overview[1]
Methyl o-bromobenzoate is an organic intermediate that can be prepared by one-step bromination of methyl benzoate.
Preparation[1]
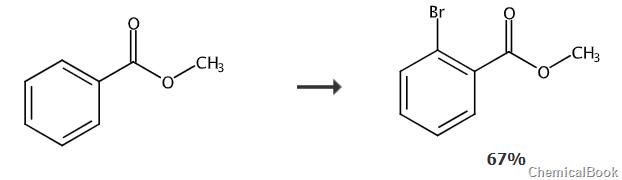
In a 250ml three-necked flask, add 0.04mol of the raw material methyl benzoate and 100ml of acetic acid, stir to dissolve, then use an ice-salt bath to cool to 0°C; dissolve 0.05mol of Br2 in 50ml In acetic acid, slowly add the acetic acid solution of bromine to the above reaction system dropwise. After the dropwise addition, control the temperature to 20-25°C, stir and react for 12 hours, and take a sample spot plate, which shows that there is no remaining raw material I1-3; after the reaction is completed, Add NaOH aqueous solution dropwise to neutralize the reaction solution, add dichloromethane to extract, separate the layers, filter the organic phase, distill the filtrate under reduced pressure until there is no fraction, pass through a neutral silica gel column, and obtain the intermediate methyl o-bromobenzoate, HPLC purity 99.3 %, yield 66.9%; elemental analysis structure (molecular formula C8H7BrO2): theoretical value C, 44.68; H, 3.28; Br, 37.16; test value: C, 44.67; H, 3.29; Br, 37.16. ESI-MS(m/z)(M+): The theoretical value is 213.96, and the measured value is 214.26.
Apply[2-3]
Methyl o-bromobenzoate can be used to prepare methyl phenylboronic acid-2-carboxylate. Phenylboronic acid-2-carboxylic acid methyl ester is an important intermediate in the preparation of new antihypertensive drugs and Losartan, a drug used to treat diabetic nephropathy. It is also an important organic synthesis intermediate and pharmaceutical and pesticide intermediate, and is widely used in Suzuki cross-coupling. reactions, asymmetric synthesis of amino acids, amino compound catalysts, etc. It is also widely used in the synthesis of drugs and the synthesis of asymmetric catalysts. It also has very important applications in sugar sensors, material separation and purification, and drug controlled release systems. CN200810154714.2 discloses a method for preparing methyl phenylboronic acid-2-carboxylate. Raw materials that have been commercialized on the market or methyl o-bromobenzoate that is easy to prepare are selected as the initial raw materials. Under low temperature conditions, normal Butyllithium is dropped into a mixture of the main raw materials methyl o-bromobenzoate and borate ester compounds, and is hydrolyzed to prepare methyl phenylboronic acid-2-carboxylate. This method has easy-to-obtain raw materials, high reaction purity and high yield, and the process is The conditions are stable, the operation is simple, and it is suitable for large-scale production. It provides a new idea and method for the preparation of phenylboronic acid-2-carboxylic acid methyl ester.
For the preparation of indolofluorene. In recent years, with the rise of the OLED field, fluorene derivatives have occupied an increasing proportion in the OLED field due to their outstanding optoelectronic properties. CN201810200173.6 discloses a preparation method of indolofluorene, which relates to the field of compound synthesis. It uses phenylboronic acid and methyl o-bromobenzoate as raw materials to prepare 1-(2-nitrophenyl)-9 through a series of reactions. , 9-dimethylfluorene; prepared by using 1-(2-nitrophenyl)-9,9-dimethylfluorene as raw material and o-dichlorobenzene as solvent through triphenylphosphorus ring-closure reaction Final product. The raw materials used in the invention are simple and easy to obtain, the cost is low, the synthesis process is simple, the yield is high, and it has great reference significance and high commercial value in the synthesis of fluorene compounds.
Main reference materials
[1] [Chinese invention] CN201710896498.8 A fluorene organic compound and its application in OLED devices
[2] [Chinese invention, Chinese invention authorization] CN200810154714.2 A method for preparing phenylboronic acid-2-carboxylic acid methyl ester
[3] CN201810200173.6 A preparation method of indolofluorene

 微信扫一扫打赏
微信扫一扫打赏

