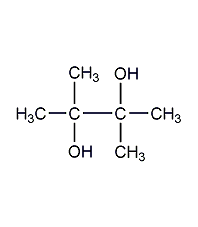
Structural formula
| Business number | 01KS |
|---|---|
| Molecular formula | C6H14O2 |
| Molecular weight | 118.17 |
| label |
Tetramethylethylene glycol, 2,3-dimethyl-2,3-butanediol, 2,3-dimethyl-2,3-dihydroxybutane, pineapple alcohol, 2,3-Dimethylbutane-2,3-diol, 2,3-Dimethyl-2,3-butanediol, Pinacone, Tetramethylethylene glycol, HOC(CH3)2C(CH3)2OH, alcohol solvents, aliphatic compounds |
Numbering system
CAS number:76-09-5
MDL number:MFCD00004462
EINECS number:200-933-5
RTECS number:EK1720000
BRN number:1340501
PubChem number:24887556
Physical property data
1. Properties: Those containing 6 molecules of crystal water are colorless flake crystals, and those that crystallize in alcohol or ether are colorless needle-like anhydrous substances.
2. Density (g/mL, 15/4℃, supercooled liquid): 0.967
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 43
5. Boiling point (ºC, 101.3kPa): 174.4
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain Determined
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit ( %, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Slightly soluble in cold water, soluble in heat Water, alcohol, ether, slightly soluble in carbon disulfide.
Toxicological data
1. Acute toxicity
Mouse caliber LD50: 3380mg/kg
2. Neurotoxicity
Rabbit skin test: 500mg/24HREACTION SEVERITY
p>
Ecological data
None
Molecular structure data
1. �� Er refractive index: 32.84
2. Molar volume (cm3/mol): 122.6
3. Isotonic specific volume (90.2K): 294.5
4. Surface tension (dyne/cm): 33.2
5. Polarizability (10-24cm3) :13.02
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0.1
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 40.5
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 72.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the stereocenter of chemical bonds Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Slightly toxic and flammable. Molecular rearrangement reactions can occur to generate 3,3-dimethyl-2-butanone.
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
1. Obtained by reduction of acetone. Put magnesium chips into industrial benzene, heat to reflux, slowly add a solution of acetone and mercuric chloride, react for 2 hours, then add water for hydrolysis for 1 hour. After the reaction is completed, cool and filter, recover benzene, and then add water, then pinacol containing crystal water will crystallize out. Add the crystal to industrial benzene for dehydration to produce anhydrous pinacol. After distilling the benzene solution of pinacol to recover benzene, the distillate at 170-175°C is then fractionated to collect the distillate to obtain pinacol.
2. Preparation method:
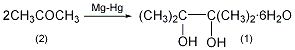
Add 20g (0.83mol) magnesium chips and 200mL of anhydrous benzene into the dry reaction bottle. Add a solution of 22.5g benzyl chloride dissolved in 100mL of dry analytical alcohol acetone into the addition funnel. First add about 25 mL and stir vigorously. If the reaction does not occur within a few minutes, heat it slightly. Once the reaction starts, stop heating immediately and cool it in a water bath. Add the remaining benzyl chloride solution while maintaining vigorous reaction. After the addition is completed, add 50g (63.5mL, 0.86mol) dry acetone (2) and 50mL benzene. After the reaction slows down, heat it in a water bath for 1 to 2 hours. At this time, magnesium pinacol precipitates and reflux for 1 hour to complete the reaction. Add 50 mL of water dropwise, heat in a water bath for 1 hour, cool to 50°C, and filter with suction. The filter cake is suspended in 125 mL of benzene, refluxed for 10 minutes, and filtered with suction. Combine the organic liquids, distill to half the volume ①, add 75 mL of water, and cool in an ice bath to precipitate solid. Filter with suction, wash with a small amount of benzene, and dry in the air to obtain 90g of pinacol hexahydrate (1)②, yield 48%, mp45.5°C. Note: ① Mainly evaporate the reacted acetone. ②The purity of the crude product can meet the requirements for the preparation of pinacolone, etc. If the pure product is made, it can be dissolved in hot water of equal mass, the activated carbon will decolorize, and the solid will precipitate after cooling, with a recovery rate of 95%. It azeotropically removes water with benzene, and then distills to collect the 169-173°C fraction. After solidification, it generates anhydrous pinacol, mp. 43°C. Slowly absorb it in the air until it forms six compounds. [1]
Purpose
Used in the synthesis of pinacolone, dimethylbutadiene, etc.

 微信扫一扫打赏
微信扫一扫打赏

