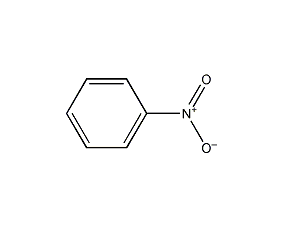
Structural formula
| Business number | 02EW |
|---|---|
| Molecular formula | C6H5NO2 |
| Molecular weight | 123.11 |
| label |
Mononitrobenzene, Miban oil, spot oil, Mirban oil, artificial bitter almond oil, Bitter almond oil, Oil of mirbane, Essence of mirbane, Mirbane oil, Nitrobenzol, Molecular polymerization inhibitor, Organic solvents |
Numbering system
CAS number:98-95-3
MDL number:MFCD00007043
EINECS number:202-716-0
RTECS number:DA6475000
BRN number:507540
PubChem number:24858237
Physical property data
1. Properties: light yellow transparent oily liquid with bitter almond smell. [1]
2. Melting point (℃): 5.7[2]
3. Boiling point (℃): 210.8 [3]
4. Relative density (water = 1): 1.20[4]
5. Relative vapor density (Air=1): 4.25[5]
6. Saturated vapor pressure (kPa): 0.02 (20℃)[6]
7. Critical pressure (MPa): 4.82[7]
8. Octanol/water partition coefficient: 1.85~1.88[8]
9. Flash point (℃): 88 (CC) [9]
10. Ignition temperature (℃): 482[10]
11. Explosion upper limit (%): 40[11]
12. Explosion lower limit (%): 1.8 (93℃)[12]
13. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol, ether, benzene, acetone and so on. [13]
14. Heat of evaporation (KJ/mol, 484K): 47.73
15. Heat of fusion (KJ/mol, 278.98K): 11.60
16. Conductivity (S/m, 23.6ºC): 1.22×10-8
17. Conductivity (S/m, 25ºC ): 9.1×10-7
18. Thermal conductivity (W/(m·K), 20ºC): 0.13649
19. Volume expansion Coefficient (K-1,0~30ºC): 0.00083
Toxicological data
1. Acute toxicity[14]
LD50: 489mg/kg (rat oral); 2100mg/kg (rat oral Skin)
2. Irritation [15]
Rabbit transdermal: 500mg (24h), mild irritation .
Rabbit eye: 500mg (24h), mild irritation.
3. Mutagenicity [16] Microbial mutagenicity: Salmonella typhimurium hamster lung 200μg/L.
4. Carcinogenicity [17] IARC Carcinogenicity Comment: G2B, suspected human carcinogen.
5. Others[18] The minimum toxic concentration for inhalation in rats (TCLo): 5ppm (6h) (90d, male), affecting sperm production , affecting the testicles, epididymis, and vas deferens.
Ecological Mathematics��
1. Ecotoxicity[19]
LC50: 27mg/L (48h) (water flea); 42.6mg/L (48h) ) (bluegill sunfish); 117mg/L (96h) (black-body minnow); 125mg/L (48h) (medaka)
IC50: 1.9~33mg/L (72h) (algae)
2. Biodegradability[20]
Aerobic biodegradation (h): 322~4728
Anaerobic biodegradation (h): 48~300
3. Non-biodegradability[21]
Photolysis half-life in water (h): 1608~4800
Photolysis maximum absorption (nm): 259
Photooxidation half-life in water (h): 3009~1.90×10 5
Half-life of photooxidation in air (h): 0.544~5.44
4. Bioaccumulation [22]
BCF: 2~4.8 (carp, exposure concentration 125ppb, exposure time 6 weeks); 1.6~7.7 (carp, exposure concentration 12.5ppb, exposure time 6 weeks)
Molecular structure data
1. Molar refractive index: 32.79
2. Molar volume (cm3/mol): 101.2
3. Isotonic specific volume (90.2K ): 262.7
4. Surface tension (dyne/cm): 45.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.00
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 102
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: relatively stable to acids and alkalis. It can evaporate with water vapor and has weak oxidizing effect. Use metals such as iron and zinc to react with hydrochloric acid, or use nickel, copper, silver, etc. as catalysts, and pressurize for reduction to generate aniline. It reacts with the mixed acid of sulfuric acid and nitric acid to produce dinitrobenzene or trinitrobenzene. Chlorination reaction in the presence of iodine or magnesium chloride produces m-chloronitrobenzene. The chlorination reaction is carried out in the presence of ferric chloride to generate 2,5-dichloronitrobenzene at room temperature. 2,3,5,6-tetrachloronitrobenzene is generated at 100℃. Hexachlorobenzene is generated above 100℃. The reaction with fuming sulfuric acid mainly produces m-nitrobenzene sulfonic acid. It reacts with potassium hydroxide to produce o-nitrophenol and a small amount of p-nitrophenol. Nitrobenzene reacts with Grignard’s reagent.
2. Stability[23] Stable
3. Incompatible substances[24] Strong oxidants, ammonia, amines, etc.
4. Polymerization hazards[25] No polymerization
5. Decomposition products[26] Nitrogen oxides
Storage method
Storage Precautions[27] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
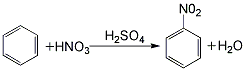
1. Obtained from nitration of benzene with mixed acid. This is the traditional method for producing nitrobenzene. It was initially produced by batch method, then developed into continuous method, and recently developed into adiabatic nitrification method. The batch method is to react benzene and mixed acid in a cast iron nitrification pot at 50°C. Continuous nitrification uses nitrifiers in series. In order to fully utilize the nitric acid and reduce the generation of dinitrobenzene, a slight excess of benzene is added. The advantage of the continuous method is that the production capacity is large and the concentration of nitric acid can be appropriately reduced. The adiabatic nitrification method can be operated intermittently or continuously, and the reaction heat can be used to concentrate sulfuric acid, with less dinitrobenzene as a by-product. Raw material consumption quota: pure benzene (industrial product) 665kg/t, nitric acid (98%) 570kg/t, sulfuric acid (92.5%) 50kg/t, soda ash 20kg/t.
2. Refining method: Nitrobenzene usually contains nitrotoluene, dinitrothiophene, dinitrothiophene, Impurities such as nitrobenzene and aniline. During purification, wash with dilute sulfuric acid, dilute alkali and water in sequence, dry with calcium chloride and repeat vacuum distillation. It can also be purified by fractional crystallization or recrystallization with absolute ethanol. Others The refining method is: steam distillation in the presence of dilute sulfuric acid, the distillate is separated from the water layer, dried with calcium chloride, and then decompressed in the presence of barium oxide, phosphorus pentoxide, aluminum trichloride or activated alumina. Distillation.
3. Use industrial nitrobenzene as raw material, add anhydrous calcium chloride to dry and remove water, and then perform distillation to collect the 208.5~212.0°C fraction to obtain the finished product. Vacuum distillation can also be used , the operating pressure is 267Pa.
Purpose
1. This product is a molecular polymerization inhibitor, and the dosage is 0.10% to 0.001%. This product is an important chemical raw material, used in the manufacture of aniline, azobenzene, dyes, etc., and also used as an organic solvent. Used as a solvent for cellulose ethers, etc. Because it can dissolve aluminum trichloride, it is widely used as a solvent for Friedel-Crafts reactions. In addition, nitrobenzene is also an important chemical raw material and can be used as an intermediate for a variety of medicines and dyes, such as aniline, dinitrobenzene, benzidine, azobenzene, meta-aminobenzene sulfonic acid, etc.
2. Used as solvent to produce aniline, dyes, etc. [28]
agent. Because it can dissolve aluminum trichloride, it is widely used as a solvent for Friedel-Crafts reactions. In addition, nitrobenzene is also an important chemical raw material and can be used as an intermediate for a variety of medicines and dyes, such as aniline, dinitrobenzene, benzidine, azobenzene, meta-aminobenzene sulfonic acid, etc.
2. Used as solvent to produce aniline, dyes, etc. [28]

 微信扫一扫打赏
微信扫一扫打赏

